Page 341 - Hydrogeology Principles and Practice
P. 341
HYDD01 12/5/05 5:31 PM Page 324
324 Appendix Five
+ + 2+ 2+ −
conservative species Na , K , Ca , Mg , Cl and waters. The resulting water vapour ultimately con-
2−
SO . Some of the less abundant, non-conservative denses to form rain, and the overall process can be
4
−
species, notably HCO , SiO and the ions of N and P, viewed as purification by natural distillation. How-
3 2
participate in biological processes and therefore show ever, solid particles and gases in the atmosphere are
widely varying concentrations depending on the dissolved in rainwater resulting in a wide range in
local abundance of marine organisms and supply of chemical composition, as well as variation in pH.
organic carbon. Seawater pH is remarkably constant, Broadly, and as shown in Tables A5.2 and A5.3, rain-
normally in the range 7.8–8.4, and is buffered prin- water species derived from terrestrial sources are
2+ + + −
cipally by inorganic reactions involving carbonate mainly dominated by Ca , K , NH and NO , and
4 3
+
−
species (Krauskopf & Bird 1995). from marine sources the main species are Cl , Na ,
2+ 2−
Mg and SO . Elements in rain that result from
4
rainout (determined by the composition of nucleat-
A5.2 Rainwater composition ing aerosols) show little change or a slight rise in
concentration with time. By contrast, elements con-
Rainwater can be described as a weakly acidic, dilute tributed by washout (determined by the composi-
solution, with a pH in the range 4–6 and a total salt tion of soluble trace gases) exhibit a sharp decrease
content of just a few milligrammes per litre. Eva- in concentration with time as the air is essentially
poration into the atmosphere results in separation cleaned during the rainfall event (Berner & Berner
of water molecules from dissolved salts in surface 1987).
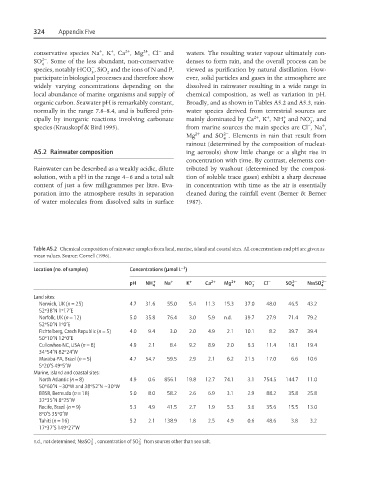
Table A5.2 Chemical composition of rainwater samples from land, marine, island and coastal sites. All concentrations and pH are given as
mean values. Source: Cornell (1996).
− −1
Location (no. of samples) Concentrations (mmol L )
pH NH 4 + + Na + + K + + Ca 2+ + Mg 2+ + NO − − 3 Cl − − SO 4 2− − NssSO 4 2− −
Land sites:
Norwich, UK (n = 25) 4.7 31.6 55.0 5.4 11.3 15.3 37.0 48.0 46.5 43.2
52º38′N 1º17′E
Norfolk, UK (n = 12) 5.0 35.8 76.4 3.0 5.9 n.d. 39.7 27.9 71.4 79.2
52º50′N 1º0′E
Fichtelberg, Czech Republic (n = 5) 4.0 9.4 3.0 2.0 4.9 2.1 10.1 8.2 39.7 39.4
50º10′N 12º0′E
Cullowhee-NC, USA (n = 8) 4.9 2.1 8.4 9.2 8.9 2.0 6.3 11.4 18.1 19.4
34º54′N 82º24′W
Maraba-PA, Brazil (n = 5) 4.7 54.7 59.5 2.9 2.1 6.2 21.5 17.0 6.6 10.6
5º20′S 49º5′W
Marine, island and coastal sites:
North Atlantic (n = 8) 4.9 0.6 856.1 19.8 12.7 74.1 3.1 754.5 144.7 11.0
50º60′N 30ºW and 38º52′N 30ºW
~
~
BBSR, Bermuda (n = 18) 5.0 8.0 58.2 2.6 6.9 3.1 2.9 88.2 35.8 25.8
32º35′N 8º25′W
Recife, Brazil (n = 9) 5.3 4.9 41.5 2.7 1.9 5.3 3.6 35.6 15.5 13.0
8º0′S 35º0′W
Tahiti (n = 16) 5.2 2.1 138.9 1.8 2.5 4.9 0.6 48.6 3.8 3.2
17º37′S 149º27′W
2−
n.d., not determined; NssSO , concentration of SO 2− from sources other than sea salt.
4
4