Page 92 - Hydrogeology Principles and Practice
P. 92
HYDC03 12/5/05 5:36 PM Page 75
Chemical hydrogeology 75
Table 3.1 The relative abundance of hydrogen and oxygen H O
2
+
isotopes in the water molecule. Na Cl − Na + + Cl − eq. 3.2
(aq) (aq)
Isotope Relative abundance Type
Positively charged atoms like sodium are known as
1 H Proteum 99.984 Stable cations, while negatively charged ions like chloride
2 H Deuterium 0.016 Stable are called anions. When writing chemical equations,
3 H Tritium 0–10 −15 Radioactive* the sum of charges on one side of the equation must
16 O Oxygen 99.76 Stable
17 O Oxygen 0.04 Stable balance the sum of charges on the other side. On the
18 O Oxygen 0.20 Stable left-hand side of equation 3.2, NaCl is an electrically
neutral compound, while on the right-hand side, the
* Half-life = 12.3 years. aqueous sodium and chloride ions each carry a single
but opposite charge so that the charges cancel, or bal-
ance, each other.
phases is known as proton transfer, an important con- The degree of hydration increases with increasing
sideration in carbonate chemistry. electrical charge of the dissociated ion and also with
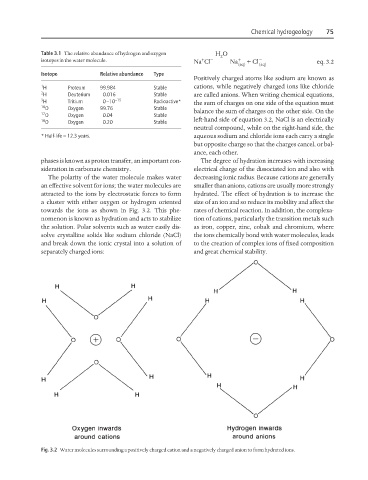
The polarity of the water molecule makes water decreasing ionic radius. Because cations are generally
an effective solvent for ions; the water molecules are smaller than anions, cations are usually more strongly
attracted to the ions by electrostatic forces to form hydrated. The effect of hydration is to increase the
a cluster with either oxygen or hydrogen oriented size of an ion and so reduce its mobility and affect the
towards the ions as shown in Fig. 3.2. This phe- rates of chemical reaction. In addition, the complexa-
nomenon is known as hydration and acts to stabilize tion of cations, particularly the transition metals such
the solution. Polar solvents such as water easily dis- as iron, copper, zinc, cobalt and chromium, where
solve crystalline solids like sodium chloride (NaCl) the ions chemically bond with water molecules, leads
and break down the ionic crystal into a solution of to the creation of complex ions of fixed composition
separately charged ions: and great chemical stability.
Fig. 3.2 Water molecules surrounding a positively charged cation and a negatively charged anion to form hydrated ions.