Page 96 - Hydrogeology Principles and Practice
P. 96
HYDC03 12/5/05 5:36 PM Page 79
Chemical hydrogeology 79
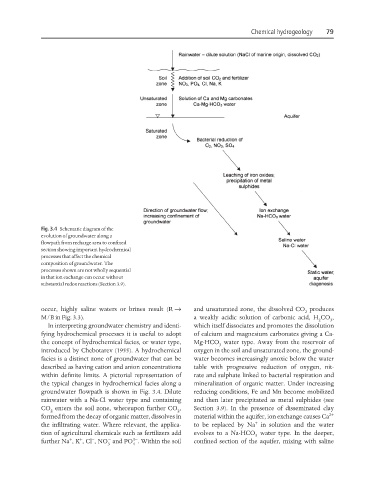
Fig. 3.4 Schematic diagram of the
evolution of groundwater along a
flowpath from recharge area to confined
section showing important hydrochemical
processes that affect the chemical
composition of groundwater. The
processes shown are not wholly sequential
in that ion exchange can occur without
substantial redox reactions (Section 3.9).
occur, highly saline waters or brines result (R → and unsaturated zone, the dissolved CO produces
2
M/B in Fig. 3.3). a weakly acidic solution of carbonic acid, H CO ,
2 3
In interpreting groundwater chemistry and identi- which itself dissociates and promotes the dissolution
fying hydrochemical processes it is useful to adopt of calcium and magnesium carbonates giving a Ca-
the concept of hydrochemical facies, or water type, Mg-HCO water type. Away from the reservoir of
3
introduced by Chebotarev (1955). A hydrochemical oxygen in the soil and unsaturated zone, the ground-
facies is a distinct zone of groundwater that can be water becomes increasingly anoxic below the water
described as having cation and anion concentrations table with progressive reduction of oxygen, nit-
within definite limits. A pictorial representation of rate and sulphate linked to bacterial respiration and
the typical changes in hydrochemical facies along a mineralization of organic matter. Under increasing
groundwater flowpath is shown in Fig. 3.4. Dilute reducing conditions, Fe and Mn become mobilized
rainwater with a Na-Cl water type and containing and then later precipitated as metal sulphides (see
CO enters the soil zone, whereupon further CO , Section 3.9). In the presence of disseminated clay
2 2
formed from the decay of organic matter, dissolves in material within the aquifer, ion exchange causes Ca 2+
+
the infiltrating water. Where relevant, the applica- to be replaced by Na in solution and the water
tion of agricultural chemicals such as fertilizers add evolves to a Na-HCO water type. In the deeper,
3
+ + − − 3−
further Na , K , Cl , NO and PO . Within the soil confined section of the aquifer, mixing with saline
3 4