Page 127 - Illustrated Pocket Dictionary of Chromatography
P. 127
126 METHOXYSILANES
ment performance evaluation, limits of detection, specificity,
selectivity, accuracy and precision, repeatability, ruggedness, and
robustness.
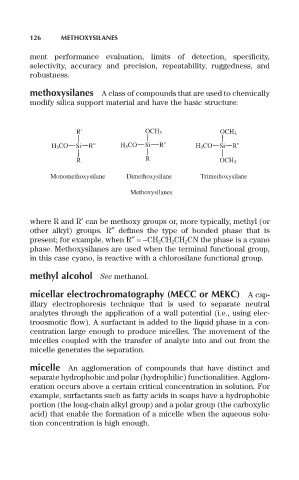
methoxysilanes A class of compounds that are used to chemically
modify silica support material and have the basic structure:
R' OCH 3 OCH 3
H 3 CO Si R'' H 3 CO Si R'' H 3 CO Si R''
R R OCH 3
Monomethoxysilane Dimethoxysilane Trimethoxysilane
Methoxysilanes
where R and R¢ can be methoxy groups or, more typically, methyl (or
other alkyl) groups. R≤ defines the type of bonded phase that is
present; for example, when R≤=-CH 2 CH 2 CH 2 CN the phase is a cyano
phase. Methoxysilanes are used when the terminal functional group,
in this case cyano, is reactive with a chlorosilane functional group.
methyl alcohol See methanol.
micellar electrochromatography (MECC or MEKC) A cap-
illary electrophoresis technique that is used to separate neutral
analytes through the application of a wall potential (i.e., using elec-
troosmotic flow). A surfactant is added to the liquid phase in a con-
centration large enough to produce micelles. The movement of the
micelles coupled with the transfer of analyte into and out from the
micelle generates the separation.
micelle An agglomeration of compounds that have distinct and
separate hydrophobic and polar (hydrophilic) functionalities. Agglom-
eration occurs above a certain critical concentration in solution. For
example, surfactants such as fatty acids in soaps have a hydrophobic
portion (the long-chain alkyl group) and a polar group (the carboxylic
acid) that enable the formation of a micelle when the aqueous solu-
tion concentration is high enough.