Page 187 - Industrial Ventilation Design Guidebook
P. 187
4,4 WATER PROPERTIES AND TREATMENT 1 49
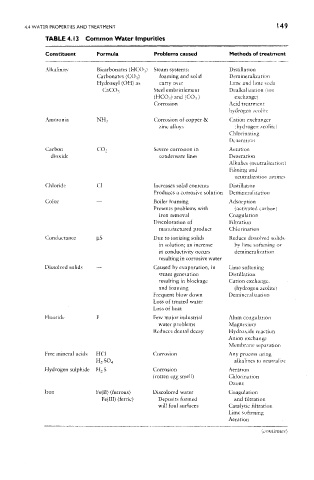
TABLE 4. i 3 Common Water Impurities
Constituent Formula Problems caused Methods of treatment
Alkalinity Bicarbonates (HCO^) Steam systems: Distillation
Carbonates (CO ? ) foaming and solid Demineralization
Hydroxyl (OH) as carry over Lime and lime soda
Steel embrittlement Dealkalization (ion
CaCO 3
(HCO 3) and (CO 3 ) exchange)
Corrosion Acid treatment
hydrogen zeolite
Ammonia NH^ Corrosion of copper & Cation exchanger
zinc alloys (hydrogen zeolite)
Chlorinating
Deaeratiori
Carbon CO 2 Severe corrosion in Aeration
dioxide condensate lines Deaeration
Alkal ies ( neutra lization !
Filming and
neutralization amines
Chloride C! Increases solid contents Distillation
Produces a corrosive solution Demineraltzation
Color — Boiler foaming Adsorption
Presents problems with (activated carbon)
iron removal Coagulation
Discoloration of Filtration
manufactured product Chtorinadon
Conductance p.S Due to ionizing solids Reduce dissolved solids
in solution; an increase by lime softening or
in conductivity occurs demineralization
resulting in corrosive water
Dissolved solids — Caused by evaporation, in Lime softening
steam generation Distillation
resulting in blockage Cation exchange.
and foaming (hydrogen zeolite)
Frequent blow down Deminera fixation
Loss of treated water
Loss of heat
Fluoride F Few major industrial Alum coagulation
water problems Magnesium
Reduces dental decay Hydroxide reaction
Amon exchange
Membrane separation
Free mineral acids HC1 Corrosion Any process using
alkalines to neutralize
H 2SO 4
Hydrogen sulphide H 2 S Corrosion Aeration
(rotten egg smell) Chlorination
Ozone
Iron Fe(II) (ferrous) Discolored water Coagulation
Fe(III) (ferric) Deposits formed and filtration
will foul surfaces Catalytic filtration
Lime softening
Aeration