Page 206 - Industrial Wastewater Treatment, Recycling and Reuse
P. 206
180 Industrial Wastewater Treatment, Recycling, and Reuse
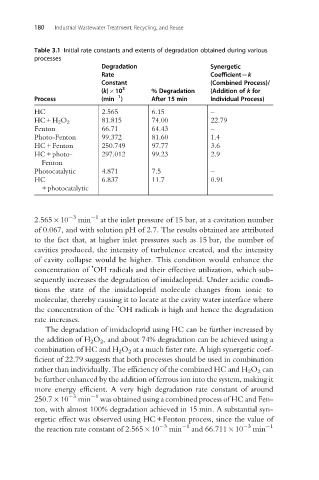
Table 3.1 Initial rate constants and extents of degradation obtained during various
processes
Degradation Synergetic
Rate Coefficient¼k
Constant (Combined Process)/
(k) 10 3 % Degradation (Addition of k for
1
Process (min ) After 15 min Individual Process)
HC 2.565 6.15 –
81.815 74.00 22.79
HC+H 2 O 2
Fenton 66.71 64.43 –
Photo-Fenton 99.372 81.60 1.4
HC+Fenton 250.749 97.77 3.6
HC+photo- 297.012 99.23 2.9
Fenton
Photocatalytic 4.871 7.5 –
HC 6.837 11.7 0.91
+photocatalytic
3 1
2.565 10 min at the inlet pressure of 15 bar, at a cavitation number
of 0.067, and with solution pH of 2.7. The results obtained are attributed
to the fact that, at higher inlet pressures such as 15 bar, the number of
cavities produced, the intensity of turbulence created, and the intensity
of cavity collapse would be higher. This condition would enhance the
•
concentration of OH radicals and their effective utilization, which sub-
sequently increases the degradation of imidacloprid. Under acidic condi-
tions the state of the imidacloprid molecule changes from ionic to
molecular, thereby causing it to locate at the cavity water interface where
•
the concentration of the OH radicals is high and hence the degradation
rate increases.
The degradation of imidacloprid using HC can be further increased by
the addition of H 2 O 2 , and about 74% degradation can be achieved using a
combination of HC and H 2 O 2 at a much faster rate. A high synergetic coef-
ficient of 22.79 suggests that both processes should be used in combination
rather than individually. The efficiency of the combined HC and H 2 O 2 can
be further enhanced by the addition of ferrous ion into the system, making it
more energy efficient. A very high degradation rate constant of around
3 1
250.7 10 min was obtained using a combined process of HC and Fen-
ton, with almost 100% degradation achieved in 15 min. A substantial syn-
ergetic effect was observed using HC+Fenton process, since the value of
the reaction rate constant of 2.565 10 3 min 1 and 66.711 10 3 min 1