Page 204 - Industrial Wastewater Treatment, Recycling and Reuse
P. 204
178 Industrial Wastewater Treatment, Recycling, and Reuse
3.6 CASE STUDIES
3.6.1 Intensification of Degradation of Imidacloprid in
Aqueous Solutions using Combination of HC with Various AOPs
Pesticides are introduced into the environment mainly through industrial
effluents, agricultural runoff, and chemical spills. The pesticides and insecti-
cides that find their way into natural bodies of water cause eutrophication and
perturbations in aquatic life and pose a major threat to the surrounding eco-
systems, mainly due to the documented health hazards caused by toxicity and
the potentially carcinogenic nature of such organic pollutants. Imidacloprid
belongstothenewclassofinsecticidecalledneonicotinoidandhashighactiv-
ity against sucking pests, including rice-hoppers, aphids, thrips, and white
flies. It has raised concerns particularly because of its possible impact on
bee populations and its potential harmful effects on the aquatic environment.
Thepresenceofimidaclopridinwaterstreamscausespotentialenvironmental
problems due to its high solubility (0.58 g/L), nonbiodegradability, and
persistent nature. Due to the toxic nature of imidacloprid, it is more resistant
to degradation using conventional biological processes and hence requires
the use of advanced oxidation methods that can degrade/mineralize the
imidacloprid molecule.
Raut-Jadhav et al. (2013) investigated the degradation of imidacloprid in
aqueous solutions using the combination of HC with various other AOPs
such as Fenton, photo-Fenton, H 2 O 2 , and photocatalytic processes. The
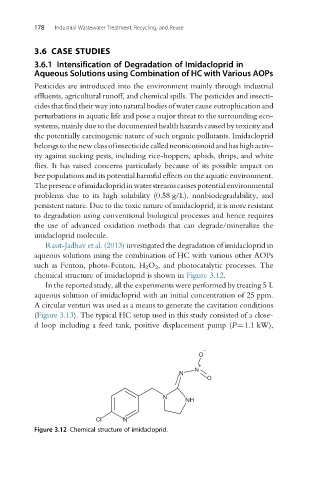
chemical structure of imidacloprid is shown in Figure 3.12.
In the reported study, all the experiments were performed by treating 5 L
aqueous solution of imidacloprid with an initial concentration of 25 ppm.
A circular venturi was used as a means to generate the cavitation conditions
(Figure 3.13). The typical HC setup used in this study consisted of a close-
d loop including a feed tank, positive displacement pump (P¼1.1 kW),
–
O
+
N
N
O
N
NH
CI N
Figure 3.12 Chemical structure of imidacloprid.