Page 202 - Industrial Wastewater Treatment, Recycling and Reuse
P. 202
176 Industrial Wastewater Treatment, Recycling, and Reuse
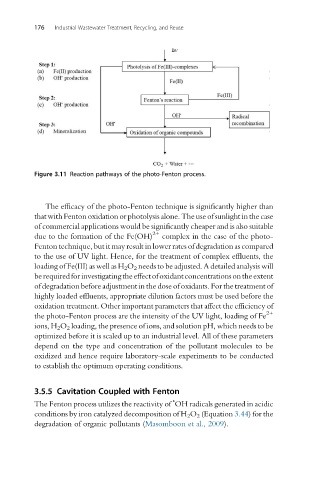
Figure 3.11 Reaction pathways of the photo-Fenton process.
The efficacy of the photo-Fenton technique is significantly higher than
that with Fenton oxidation or photolysis alone. The use of sunlight in the case
of commercial applications would be significantly cheaper and is also suitable
2+
due to the formation of the Fe(OH) complex in the case of the photo-
Fenton technique, but it may result in lower rates of degradation as compared
to the use of UV light. Hence, for the treatment of complex effluents, the
loading of Fe(III) as well as H 2 O 2 needs to be adjusted. A detailed analysis will
berequiredforinvestigatingtheeffectofoxidantconcentrationsontheextent
of degradation before adjustment in the dose of oxidants. For the treatment of
highly loaded effluents, appropriate dilution factors must be used before the
oxidation treatment. Other important parameters that affect the efficiency of
the photo-Fenton process are the intensity of the UV light, loading of Fe 2+
ions, H 2 O 2 loading, the presence of ions, and solution pH, which needs to be
optimized before it is scaled up to an industrial level. All of these parameters
depend on the type and concentration of the pollutant molecules to be
oxidized and hence require laboratory-scale experiments to be conducted
to establish the optimum operating conditions.
3.5.5 Cavitation Coupled with Fenton
•
The Fenton process utilizes the reactivity of OH radicals generated in acidic
conditions by iron catalyzed decomposition of H 2 O 2 (Equation 3.44) for the
degradation of organic pollutants (Masomboon et al., 2009).