Page 170 - Instant notes
P. 170
Physical chemistry 156
Experimental methods
Chemical kinetics is the study of the rate at which chemical reactions occur. Although
the timescales of chemical reactions vary enormously, ranging from days or years to just
a few femtoseconds (10 −15 s), the basic principle of all experimental kinetic methods is
the same. Reactants of particular concentration are brought together and some measure is
made of the rate at which the composition changes as the reaction progresses. Depending
on the specificity of the detection method available, monitoring the rate of reaction may
involve measurement of the rate at which specific reactants (or a subset of reactants) are
consumed and/or the rate at which specific products (or a subset of products) are formed,
or simply measurement of some bulk property of the system such as pressure, pH or ionic
conductivity. More sophisticated detection might involve chromatography, mass
spectrometry, or optical techniques such as absorption, fluorescence or polarimetry.
In a real-time method the composition of the system is analyzed while the reaction is
in progress, either by direct observation on the mixture or by withdrawing a small sample
and analyzing it. In the latter case it is important that analysis is fast compared with the
rate of continuing reaction. Alternatively the composition of the bulk (or of a sample
withdrawn from it) may be analyzed after the reaction has been deliberately stopped or
quenched. Quenching might be achieved by diluting the mixture rapidly in excess
solvent, neutralizing with acid or sudden cooling. Quenching is only suitable for reactions
which are slow enough for there to be little reaction during the time it takes to quench the
mixture.
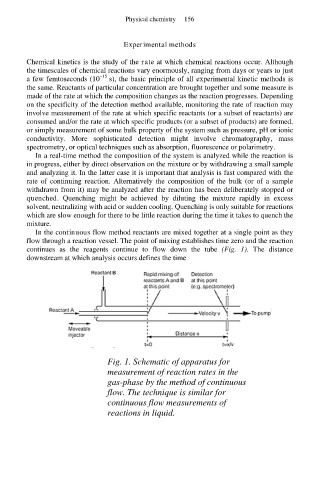
In the continuous flow method reactants are mixed together at a single point as they
flow through a reaction vessel. The point of mixing establishes time zero and the reaction
continues as the reagents continue to flow down the tube (Fig. 1). The distance
downstream at which analysis occurs defines the time
Fig. 1. Schematic of apparatus for
measurement of reaction rates in the
gas-phase by the method of continuous
flow. The technique is similar for
continuous flow measurements of
reactions in liquid.