Page 171 - Instant notes
P. 171
Empirical approaches to kinetics 157
since reaction initiation and this can be varied either by moving the point of analysis or
the point of mixing of the reagents. A disadvantage of continuous flow is that it uses a
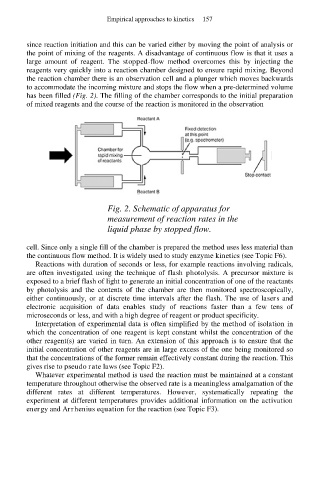
large amount of reagent. The stopped-flow method overcomes this by injecting the
reagents very quickly into a reaction chamber designed to ensure rapid mixing. Beyond
the reaction chamber there is an observation cell and a plunger which moves backwards
to accommodate the incoming mixture and stops the flow when a pre-determined volume
has been filled (Fig. 2). The filling of the chamber corresponds to the initial preparation
of mixed reagents and the course of the reaction is monitored in the observation
Fig. 2. Schematic of apparatus for
measurement of reaction rates in the
liquid phase by stopped flow.
cell. Since only a single fill of the chamber is prepared the method uses less material than
the continuous flow method. It is widely used to study enzyme kinetics (see Topic F6).
Reactions with duration of seconds or less, for example reactions involving radicals,
are often investigated using the technique of flash photolysis. A precursor mixture is
exposed to a brief flash of light to generate an initial concentration of one of the reactants
by photolysis and the contents of the chamber are then monitored spectroscopically,
either continuously, or at discrete time intervals after the flash. The use of lasers and
electronic acquisition of data enables study of reactions faster than a few tens of
microseconds or less, and with a high degree of reagent or product specificity.
Interpretation of experimental data is often simplified by the method of isolation in
which the concentration of one reagent is kept constant whilst the concentration of the
other reagent(s) are varied in turn. An extension of this approach is to ensure that the
initial concentration of other reagents are in large excess of the one being monitored so
that the concentrations of the former remain effectively constant during the reaction. This
gives rise to pseudo rate laws (see Topic F2).
Whatever experimental method is used the reaction must be maintained at a constant
temperature throughout otherwise the observed rate is a meaningless amalgamation of the
different rates at different temperatures. However, systematically repeating the
experiment at different temperatures provides additional information on the activation
energy and Arrhenius equation for the reaction (see Topic F3).