Page 176 - Instant notes
P. 176
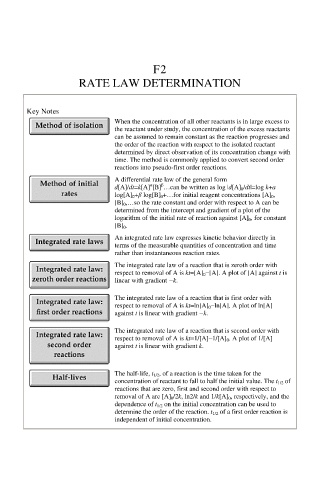
F2
RATE LAW DETERMINATION
Key Notes
When the concentration of all other reactants is in large excess to
the reactant under study, the concentration of the excess reactants
can be assumed to remain constant as the reaction progresses and
the order of the reaction with respect to the isolated reactant
determined by direct observation of its concentration change with
time. The method is commonly applied to convert second order
reactions into pseudo-first order reactions.
A differential rate law of the general form
β
α
d[A]/dt=k[A] [B] …can be written as log |d[A] 0 /dt|=log k+a
log[A] 0 +β log[B] 0 +…for initial reagent concentrations [A] 0 ,
[B] 0 ,…so the rate constant and order with respect to A can be
determined from the intercept and gradient of a plot of the
logarithm of the initial rate of reaction against [A] 0 , for constant
[B] 0 .
An integrated rate law expresses kinetic behavior directly in
terms of the measurable quantities of concentration and time
rather than instantaneous reaction rates.
The integrated rate law of a reaction that is zeroth order with
respect to removal of A is kt=[A] 0 −[A]. A plot of [A] against t is
linear with gradient −k.
The integrated rate law of a reaction that is first order with
respect to removal of A is kt=ln[A] 0 −ln[A]. A plot of ln[A]
against t is linear with gradient −k.
The integrated rate law of a reaction that is second order with
respect to removal of A is kt=1/[A]−1/[A] 0 . A plot of 1/[A]
against t is linear with gradient k.
The half-life, t 1/2 , of a reaction is the time taken for the
concentration of reactant to fall to half the initial value. The t 1/2 of
reactions that are zero, first and second order with respect to
removal of A are [A] 0 /2k, ln2/k and 1/k[A] 0 , respectively, and the
dependence of t 1/2 on the initial concentration can be used to
determine the order of the reaction. t 1/2 of a first order reaction is
independent of initial concentration.