Page 179 - Instant notes
P. 179
Rate law determination 165
The determination of a rate law using the method of initial rates has a number of
disadvantages:
(i) determining tangents to a concentration versus time plot is generally subject to
considerable uncertainty.
(ii) the rate constant is obtained by extrapolation to an intercept which increases the error
in k.
(iii) observing only initial rates may be misleading if the rate of reaction is also affected
by the formation of products. The rate constant and order may be correct at times close
to t=0 but may not be valid over the whole course of the reaction. An example is the
reaction between hydrogen and bromine to form HBr which has the rate law (see
Topic F6) of:
Initially, when [HBr] is small, but as the reaction progresses the
significance of the k′[HBr] term increases and the order with respect to Br 2 becomes
undefined.
Integrated rate laws
Reaction rates are rarely measured directly because of the difficulty in determining
accurate values for slopes of graphs. Instead an integrated rate law may be used which
expresses kinetic behavior directly in terms of the measurable observables of
concentration and time. Analytical expressions for integrated rate laws of simple types of
reaction are presented in Table 1 but even the most complex rate laws can usually be
integrated numerically by computers. Table 1 contains expressions of integrated rate laws
in terms of concentration of reactant A at time t but similar analytical expressions are
readily derived for concentration of product P at time t. The advantage of these simple
integrated rate laws is that the order of reaction with respect to a species is readily tested
by means of a suitable plot of species concentration and time (Fig. 2).
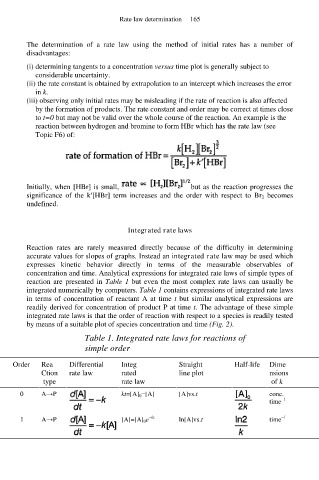
Table 1. Integrated rate laws for reactions of
simple order
Order Rea Differential Integ Straight Half-life Dime
Ction rate law rated line plot nsions
type rate law of k
0 A→P kt=[A] 0 −[A] [A]vs.t conc.
−1
time
−1
1 A→P [A]=[A] 0 e −kt ln[A]vs.t time