Page 178 - Instant notes
P. 178
Physical chemistry 164
The order, α, of the reaction with respect to A is obtained from the slope of the graph of
logarithm of initial rate of reaction of A against the logarithm of the corresponding
starting concentration [A] 0 whilst values of [B] 0,…are held constant. Similarly, the value
of β is obtained by varying only [B] 0 and measuring the initial rate of reaction of B.
In the simpler situation in which the rate law involves only a single species:
the logarithmic form (for all times) is:
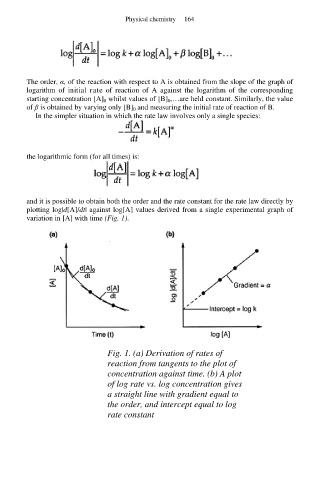
and it is possible to obtain both the order and the rate constant for the rate law directly by
plotting log|d[A]/dt| against log[A] values derived from a single experimental graph of
variation in [A] with time (Fig. 1).
Fig. 1. (a) Derivation of rates of
reaction from tangents to the plot of
concentration against time. (b) A plot
of log rate vs. log concentration gives
a straight line with gradient equal to
the order, and intercept equal to log
rate constant