Page 180 - Instant notes
P. 180
Physical chemistry 166
or
−1
−1
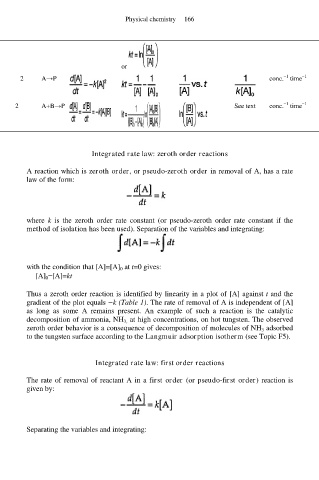
2 A→P conc. time
−1
−1
2 A+B→P See text conc. time
Integrated rate law: zeroth order reactions
A reaction which is zeroth order, or pseudo-zeroth order in removal of A, has a rate
law of the form:
where k is the zeroth order rate constant (or pseudo-zeroth order rate constant if the
method of isolation has been used). Separation of the variables and integrating:
with the condition that [A]=[A] 0 at t=0 gives:
[A] 0−[A]=kt
Thus a zeroth order reaction is identified by linearity in a plot of [A] against t and the
gradient of the plot equals −k (Table 1). The rate of removal of A is independent of [A]
as long as some A remains present. An example of such a reaction is the catalytic
decomposition of ammonia, NH 3, at high concentrations, on hot tungsten. The observed
zeroth order behavior is a consequence of decomposition of molecules of NH 3 adsorbed
to the tungsten surface according to the Langmuir adsorption isotherm (see Topic F5).
Integrated rate law: first order reactions
The rate of removal of reactant A in a first order (or pseudo-first order) reaction is
given by:
Separating the variables and integrating: