Page 172 - Instant notes
P. 172
Physical chemistry 158
Rate of reaction
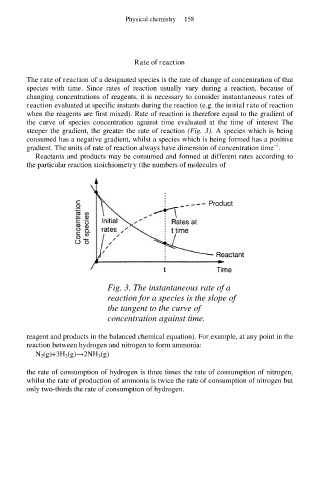
The rate of reaction of a designated species is the rate of change of concentration of that
species with time. Since rates of reaction usually vary during a reaction, because of
changing concentrations of reagents, it is necessary to consider instantaneous rates of
reaction evaluated at specific instants during the reaction (e.g. the initial rate of reaction
when the reagents are first mixed). Rate of reaction is therefore equal to the gradient of
the curve of species concentration against time evaluated at the time of interest The
steeper the gradient, the greater the rate of reaction (Fig. 3). A species which is being
consumed has a negative gradient, whilst a species which is being formed has a positive
−1
gradient. The units of rate of reaction always have dimension of concentration time .
Reactants and products may be consumed and formed at different rates according to
the particular reaction stoichiometry (the numbers of molecules of
Fig. 3. The instantaneous rate of a
reaction for a species is the slope of
the tangent to the curve of
concentration against time.
reagent and products in the balanced chemical equation). For example, at any point in the
reaction between hydrogen and nitrogen to form ammonia:
N 2(g)+3H 2(g)→2NH 3(g)
the rate of consumption of hydrogen is three times the rate of consumption of nitrogen,
whilst the rate of production of ammonia is twice the rate of consumption of nitrogen but
only two-thirds the rate of consumption of hydrogen.