Page 220 - Instant notes
P. 220
Physical chemistry 206
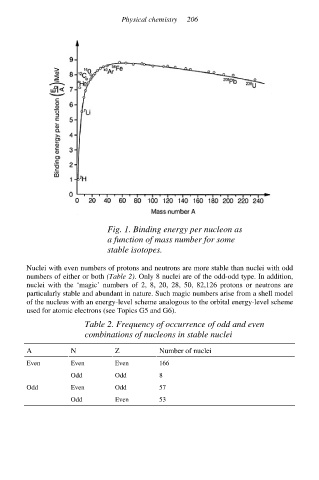
Fig. 1. Binding energy per nucleon as
a function of mass number for some
stable isotopes.
Nuclei with even numbers of protons and neutrons are more stable than nuclei with odd
numbers of either or both (Table 2). Only 8 nuclei are of the odd-odd type. In addition,
nuclei with the ‘magic’ numbers of 2, 8, 20, 28, 50, 82,126 protons or neutrons are
particularly stable and abundant in nature. Such magic numbers arise from a shell model
of the nucleus with an energy-level scheme analogous to the orbital energy-level scheme
used for atomic electrons (see Topics G5 and G6).
Table 2. Frequency of occurrence of odd and even
combinations of nucleons in stable nuclei
A N Z Number of nuclei
Even Even Even 166
Odd Odd 8
Odd Even Odd 57
Odd Even 53