Page 225 - Instant notes
P. 225
Applications of nuclear structure 211
so C−H bond breaking, which must occur at some stage in the overall process, cannot be
involved in the rate-determining step.
Effects arising from substitution of an atom directly constituting a bond that is broken
or formed during the rate determining step are known as primary kinetic isotope effects,
whereas secondary kinetic isotope effects arise from isotopic substitution elsewhere in
the molecule.
Isotope labeling
A useful application of both stable and radioactive isotopes is as tracers to identify
specific molecules in order to elucidate chemical or biochemical reaction mechanisms.
32
For example, the radioactive isotope of phosphorus, P, is routinely used during the
analysis of DNA or RNA molecules, both of which contain phosphate linkages within the
32
polymer chain. The P tag is introduced into the DNA or RNA molecules from donor
32 P-labeled molecules using specific phosphoryl transfer catalyzing enzymes. The DNA
or RNA molecules are then cleaved at specific sites using another enzyme and the
resulting mixture of fragments separated along a polyacrylamide gel by electrophoresis
(see Topic E8). The position of bands which contain the labeled DNA are determined by
the darkening of a photographic plate from the electrons emitted during the β-decay. The
sensitivity towards detection of radioactivity means that only very small amounts of
radioactive material are required for analysis.
14
The detection of radioactive C, with half-life of 5370 years, is used extensively to
trace metabolic pathways in cells. It is also used to determine the age of ancient natural
14
materials. In this application the source of C is the continual natural transmutation of
nitrogen in the atmosphere by cosmic rays. The metabolic processes in living material
14
maintain an equilibrium in the fraction of C present within the carbon of the plant or
animal. When the plant or animal dies there is no more active exchange with the source
14
14
of C and the fraction of C remaining in the material decreases at a rate determined by
its half-life.
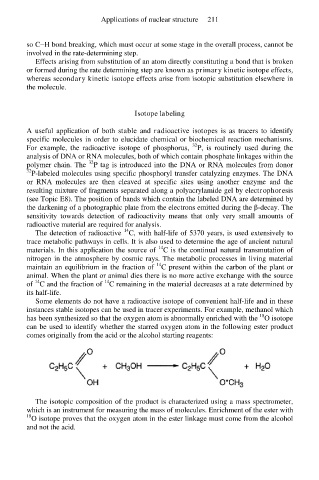
Some elements do not have a radioactive isotope of convenient half-life and in these
instances stable isotopes can be used in tracer experiments. For example, methanol which
18
has been synthesized so that the oxygen atom is abnormally enriched with the O isotope
can be used to identify whether the starred oxygen atom in the following ester product
comes originally from the acid or the alcohol starting reagents:
The isotopic composition of the product is characterized using a mass spectrometer,
which is an instrument for measuring the mass of molecules. Enrichment of the ester with
18 O isotope proves that the oxygen atom in the ester linkage must come from the alcohol
and not the acid.