Page 228 - Instant notes
P. 228
Physical chemistry 214
14
(frequency, v=c/λ=9.993×10 Hz) has an energy of 6.62×10 −19 J. One mole of these
23
photons has an energy 6.022×10 ×6.62×10 −19 = 399 kJ which exceeds the energy of
many chemical bonds and explains why ultraviolet radiation can damage molecules in
materials and biological cells.
The ultraviolet catastrophe
The attempt to derive an expression for the power emitted by a black body as a function
of wavelength was an early example of the failure of classical physics. A black body is a
perfect emitter and absorber of electromagnetic radiation, capable of emitting and
absorbing all frequencies of radiation uniformly. A good approximation of a black body
is a pinhole in a container maintained at a uniform temperature.
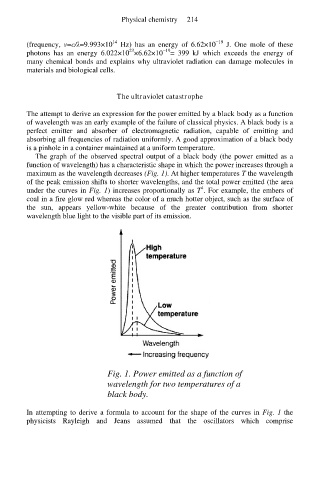
The graph of the observed spectral output of a black body (the power emitted as a
function of wavelength) has a characteristic shape in which the power increases through a
maximum as the wavelength decreases (Fig. 1). At higher temperatures T the wavelength
of the peak emission shifts to shorter wavelengths, and the total power emitted (the area
4
under the curves in Fig. 1) increases proportionally as T . For example, the embers of
coal in a fire glow red whereas the color of a much hotter object, such as the surface of
the sun, appears yellow-white because of the greater contribution from shorter
wavelength blue light to the visible part of its emission.
Fig. 1. Power emitted as a function of
wavelength for two temperatures of a
black body.
In attempting to derive a formula to account for the shape of the curves in Fig. 1 the
physicists Rayleigh and Jeans assumed that the oscillators which comprise