Page 224 - Instant notes
P. 224
Physical chemistry 210
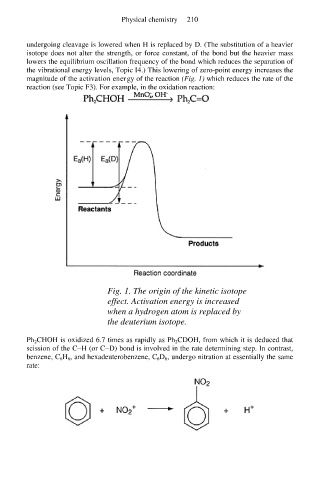
undergoing cleavage is lowered when H is replaced by D. (The substitution of a heavier
isotope does not alter the strength, or force constant, of the bond but the heavier mass
lowers the equilibrium oscillation frequency of the bond which reduces the separation of
the vibrational energy levels, Topic I4.) This lowering of zero-point energy increases the
magnitude of the activation energy of the reaction (Fig. 1) which reduces the rate of the
reaction (see Topic F3). For example, in the oxidation reaction:
Fig. 1. The origin of the kinetic isotope
effect. Activation energy is increased
when a hydrogen atom is replaced by
the deuterium isotope.
Ph 2CHOH is oxidized 6.7 times as rapidly as Ph 2CDOH, from which it is deduced that
scission of the C−H (or C−D) bond is involved in the rate determining step. In contrast,
benzene, C 6H 6, and hexadeuterobenzene, C 6D 6, undergo nitration at essentially the same
rate: