Page 223 - Instant notes
P. 223
Applications of nuclear structure 209
of the daughter thorium-234 isotope from the α-decay of uranium-238 is accompanied by
emission of γ-rays:
Half-life
It is not possible to predict exactly when an individual radioactive nucleus will
spontaneously undergo radioactive decay. However, in a sample of such nuclei it is
always the case that a fixed proportion of the sample will undergo radioactive decay
within a fixed time-span. The time taken for half the sample to decay is known as the
half-life. Since radioactive decay is a first order process the kinetics of the decay are
described by first order kinetics (see Topic F2). The half-lives of different radioactive
isotopes can vary between fractions of a second to billions of years. A selection of
important radioisotopes with their decay processes and half-lives is given in Table 1.
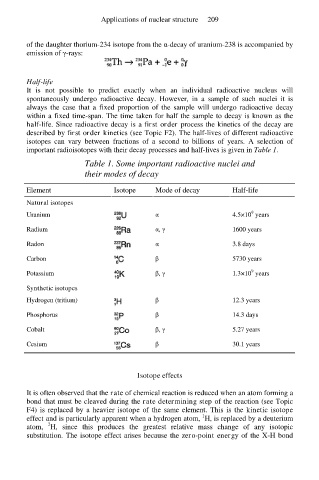
Table 1. Some important radioactive nuclei and
their modes of decay
Element Isotope Mode of decay Half-life
Natural isotopes
9
Uranium α 4.5×10 years
Radium α, γ 1600 years
Radon α 3.8 days
Carbon β 5730 years
9
Potassium β, γ 1.3×10 years
Synthetic isotopes
Hydrogen (tritium) β 12.3 years
Phosphorus β 14.3 days
Cobalt β, γ 5.27 years
Cesium β 30.1 years
Isotope effects
It is often observed that the rate of chemical reaction is reduced when an atom forming a
bond that must be cleaved during the rate determining step of the reaction (see Topic
F4) is replaced by a heavier isotope of the same element. This is the kinetic isotope
1
effect and is particularly apparent when a hydrogen atom, H, is replaced by a deuterium
2
atom, H, since this produces the greatest relative mass change of any isotopic
substitution. The isotope effect arises because the zero-point energy of the X-H bond