Page 232 - Instant notes
P. 232
Physical chemistry 218
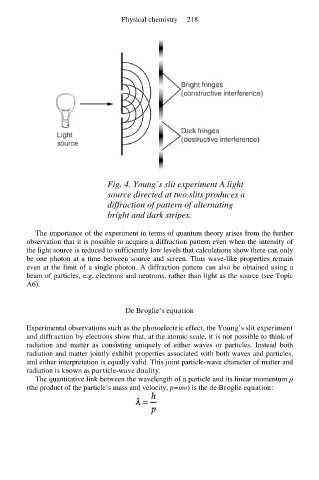
Fig. 4. Young’s slit experiment A light
source directed at two slits produces a
diffraction of pattern of alternating
bright and dark stripes.
The importance of the experiment in terms of quantum theory arises from the further
observation that it is possible to acquire a diffraction pattern even when the intensity of
the light source is reduced to sufficiently low levels that calculations show there can only
be one photon at a time between source and screen. Thus wave-like properties remain
even at the limit of a single photon. A diffraction pattern can also be obtained using a
beam of particles, e.g. electrons and neutrons, rather than light as the source (see Topic
A6).
De Broglie’s equation
Experimental observations such as the photoelectric effect, the Young’s slit experiment
and diffraction by electrons show that, at the atomic scale, it is not possible to think of
radiation and matter as consisting uniquely of either waves or particles. Instead both
radiation and matter jointly exhibit properties associated with both waves and particles,
and either interpretation is equally valid. This joint particle-wave character of matter and
radiation is known as particle-wave duality.
The quantitative link between the wavelength of a particle and its linear momentum p
(the product of the particle’s mass and velocity, p=mυ) is the de Broglie equation: