Page 236 - Instant notes
P. 236
Physical chemistry 222
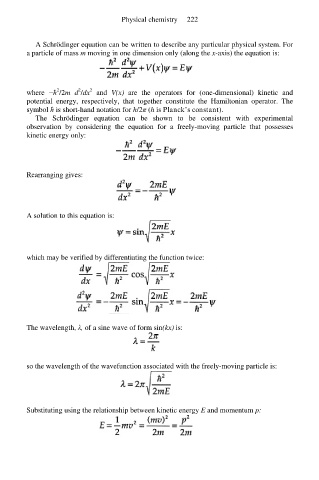
A Schrödinger equation can be written to describe any particular physical system. For
a particle of mass m moving in one dimension only (along the x-axis) the equation is:
2
2
2
where −ћ /2m d /dx and V(x) are the operators for (one-dimensional) kinetic and
potential energy, respectively, that together constitute the Hamiltonian operator. The
symbol ħ is short-hand notation for h/2π (h is Planck’s constant).
The Schrödinger equation can be shown to be consistent with experimental
observation by considering the equation for a freely-moving particle that possesses
kinetic energy only:
Rearranging gives:
A solution to this equation is:
which may be verified by differentiating the function twice:
The wavelength, λ, of a sine wave of form sin(kx) is:
so the wavelength of the wavefunction associated with the freely-moving particle is:
Substituting using the relationship between kinetic energy E and momentum p: