Page 240 - Instant notes
P. 240
Physical chemistry 226
The subscript n is added to emphasize the fact that the particle in the box is only
permitted to possess discreet values, or quanta, of E, corresponding to integer values of n
in the above expression. The allowed energies of a system are called the energy levels.
The wavefunctions associated with these energies are:
The constant a is chosen so that the total probability of finding the particle in the region
from x=0 to x=L is 1, which gives .
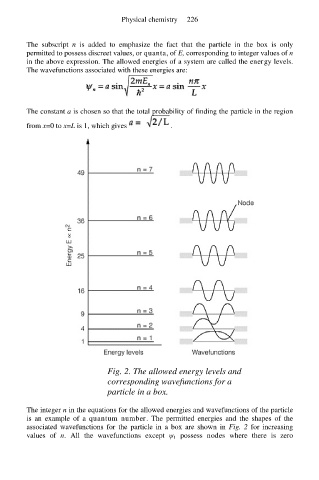
Fig. 2. The allowed energy levels and
corresponding wavefunctions for a
particle in a box.
The integer n in the equations for the allowed energies and wavefunctions of the particle
is an example of a quantum number. The permitted energies and the shapes of the
associated wavefunctions for the particle in a box are shown in Fig. 2 for increasing
values of n. All the wavefunctions except ψ 1 possess nodes where there is zero