Page 241 - Instant notes
P. 241
The wave nature of matter 227
probability of finding the particle at these points. Whilst the most probable location for a
particle with one quantum of energy is exactly midway between the walls, a particle with
two quanta of energy has zero probability of being located at that point.
The separation of adjacent energy levels is:
This expression illustrates two general features of quantum mechanical descriptions of
physical systems:
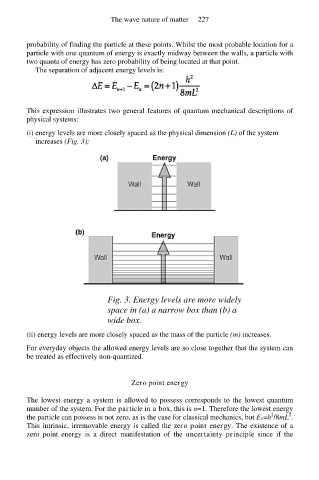
(i) energy levels are more closely spaced as the physical dimension (L) of the system
increases (Fig. 3);
Fig. 3. Energy levels are more widely
space in (a) a narrow box than (b) a
wide box.
(ii) energy levels are more closely spaced as the mass of the particle (m) increases.
For everyday objects the allowed energy levels are so close together that the system can
be treated as effectively non-quantized.
Zero point energy
The lowest energy a system is allowed to possess corresponds to the lowest quantum
number of the system. For the particle in a box, this is n=1. Therefore the lowest energy
2
2
the particle can possess is not zero, as is the case for classical mechanics, but E 1=h /8mL .
This intrinsic, irremovable energy is called the zero point energy. The existence of a
zero point energy is a direct manifestation of the uncertainty principle since if the