Page 246 - Instant notes
P. 246
Physical chemistry 232
Hydrogen spectrum: Rydberg series
A hydrogen atom emission spectrum is obtained by passing an electric discharge
through a tube of low-pressure hydrogen gas (to form excited hydrogen atoms) and
dispersing the emitted light into its constituent wavelengths using a prism or diffraction
grating. The resulting spectrum consists of light emitted at discrete frequencies only. The
emitted frequencies, v, occur in distinct groups with a regular pattern in different regions
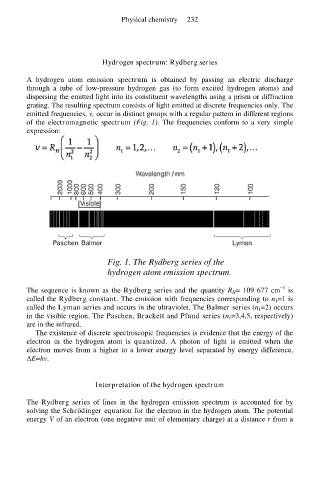
of the electromagnetic spectrum (Fig. 1). The frequencies conform to a very simple
expression:
Fig. 1. The Rydberg series of the
hydrogen atom emission spectrum.
−1
The sequence is known as the Rydberg series and the quantity R H= 109 677 cm is
called the Rydberg constant. The emission with frequencies corresponding to n 1=1 is
called the Lyman series and occurs in the ultraviolet. The Balmer series (n 1=2) occurs
in the visible region. The Paschen, Brackett and Pfund series (n 1=3,4,5, respectively)
are in the infrared.
The existence of discrete spectroscopic frequencies is evidence that the energy of the
electron in the hydrogen atom is quantized. A photon of light is emitted when the
electron moves from a higher to a lower energy level separated by energy difference,
∆E=hv.
Interpretation of the hydrogen spectrum
The Rydberg series of lines in the hydrogen emission spectrum is accounted for by
solving the Schrödinger equation for the electron in the hydrogen atom. The potential
energy V of an electron (one negative unit of elementary charge) at a distance r from a