Page 248 - Instant notes
P. 248
Physical chemistry 234
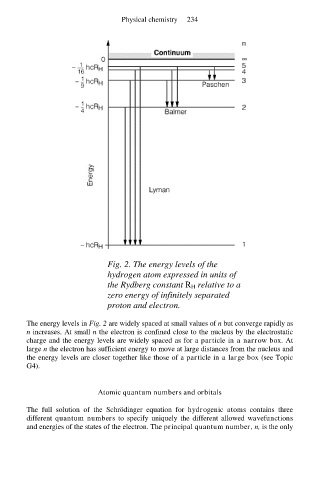
Fig. 2. The energy levels of the
hydrogen atom expressed in units of
the Rydberg constant R H relative to a
zero energy of infinitely separated
proton and electron.
The energy levels in Fig. 2 are widely spaced at small values of n but converge rapidly as
n increases. At small n the electron is confined close to the nucleus by the electrostatic
charge and the energy levels are widely spaced as for a particle in a narrow box. At
large n the electron has sufficient energy to move at large distances from the nucleus and
the energy levels are closer together like those of a particle in a large box (see Topic
G4).
Atomic quantum numbers and orbitals
The full solution of the Schrödinger equation for hydrogenic atoms contains three
different quantum numbers to specify uniquely the different allowed wavefunctions
and energies of the states of the electron. The principal quantum number, n, is the only