Page 250 - Instant notes
P. 250
Physical chemistry 236
l=0 2s 0 2
n=1 l=0 1s 0 2 2
Only hydrogenic atoms have sub-shells that are degenerate (of the same energy). For
many-electron atoms the energy of the orbital (wavefunction) depends on both n and l
so each sub-shell has different energy (Topic G6).
Shapes of atomic orbitals
In general, the mathematical equation of each atomic wavefunction contains a radial
part, describing the value of the wavefunction as a function of radial distance from the
center of the atom, and an angular part, describing the value of the wavefunction as a
function of all angles about the center, i.e., the value of the wavefunction at all points on
the surface of the sphere at a given radius, r.
For the 1s orbital (n=1, l=0, m l=0) the mathematical form of the wavefunction is:
where a 0 is a constant known as the Bohr radius. The wavefunction contains no angular
dependence so it has the same shape (an exponential decrease) in all directions from the
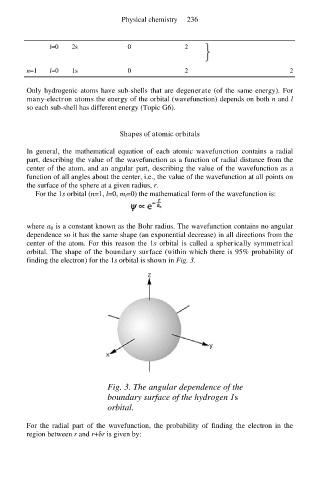
center of the atom. For this reason the 1s orbital is called a spherically symmetrical
orbital. The shape of the boundary surface (within which there is 95% probability of
finding the electron) for the 1s orbital is shown in Fig. 3.
Fig. 3. The angular dependence of the
boundary surface of the hydrogen 1s
orbital.
For the radial part of the wavefunction, the probability of finding the electron in the
region between r and r+δr is given by: