Page 252 - Instant notes
P. 252
Physical chemistry 238
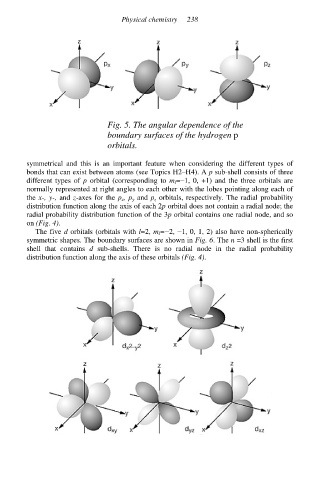
Fig. 5. The angular dependence of the
boundary surfaces of the hydrogen p
orbitals.
symmetrical and this is an important feature when considering the different types of
bonds that can exist between atoms (see Topics H2–H4). A p sub-shell consists of three
different types of p orbital (corresponding to m l=−1, 0, +1) and the three orbitals are
normally represented at right angles to each other with the lobes pointing along each of
the x-, y-, and z-axes for the p x, p y and p z orbitals, respectively. The radial probability
distribution function along the axis of each 2p orbital does not contain a radial node; the
radial probability distribution function of the 3p orbital contains one radial node, and so
on (Fig. 4).
The five d orbitals (orbitals with l=2, m l=−2, −1, 0, 1, 2) also have non-spherically
symmetric shapes. The boundary surfaces are shown in Fig. 6. The n =3 shell is the first
shell that contains d sub-shells. There is no radial node in the radial probability
distribution function along the axis of these orbitals (Fig. 4).