Page 237 - Instant notes
P. 237
The wave nature of matter 223
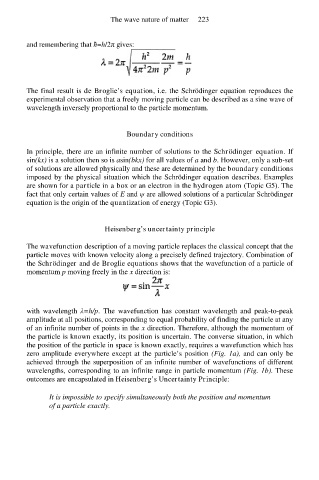
and remembering that ħ=h/2π gives:
The final result is de Broglie’s equation, i.e. the Schrödinger equation reproduces the
experimental observation that a freely moving particle can be described as a sine wave of
wavelength inversely proportional to the particle momentum.
Boundary conditions
In principle, there are an infinite number of solutions to the Schrödinger equation. If
sin(kx) is a solution then so is asin(bkx) for all values of a and b. However, only a sub-set
of solutions are allowed physically and these are determined by the boundary conditions
imposed by the physical situation which the Schrödinger equation describes. Examples
are shown for a particle in a box or an electron in the hydrogen atom (Topic G5). The
fact that only certain values of E and ψ are allowed solutions of a particular Schrödinger
equation is the origin of the quantization of energy (Topic G3).
Heisenberg’s uncertainty principle
The wavefunction description of a moving particle replaces the classical concept that the
particle moves with known velocity along a precisely defined trajectory. Combination of
the Schrödinger and de Broglie equations shows that the wavefunction of a particle of
momentum p moving freely in the x direction is:
with wavelength λ=h/p. The wavefunction has constant wavelength and peak-to-peak
amplitude at all positions, corresponding to equal probability of finding the particle at any
of an infinite number of points in the x direction. Therefore, although the momentum of
the particle is known exactly, its position is uncertain. The converse situation, in which
the position of the particle in space is known exactly, requires a wavefunction which has
zero amplitude everywhere except at the particle’s position (Fig. 1a), and can only be
achieved through the superposition of an infinite number of wavefunctions of different
wavelengths, corresponding to an infinite range in particle momentum (Fig. 1b). These
outcomes are encapsulated in Heisenberg’s Uncertainty Principle:
It is impossible to specify simultaneously both the position and momentum
of a particle exactly.