Page 230 - Instant notes
P. 230
Physical chemistry 216
(iii) the kinetic energy of the ejected electrons does not depend on the intensity of the
incident radiation. Only the number of ejected electrons depends on the intensity.
These observations cannot be reconciled with the classical interpretation of
electromagnetic waves in which the energy of a wave is assumed to depend on its
amplitude and not on its frequency.
As with the ultraviolet catastrophe, the photoelectric effect is explained by the
postulate that the energy of the incoming electromagnetic radiation is quantized such
that radiation of frequency v consists only of quanta of energy, E= hv. Therefore the
energy of the radiation depends only on the value of v; increasing the intensity of
radiation at this frequency increases the number of quanta present (n=E/hv) but does not
change the energy of each quantum. The quanta of electromagnetic radiation are called
photons. If it is assumed that each metal has a characteristic energy barrier for ejection of
an electron (called the work function, Φ) then only radiation with quanta of energy that
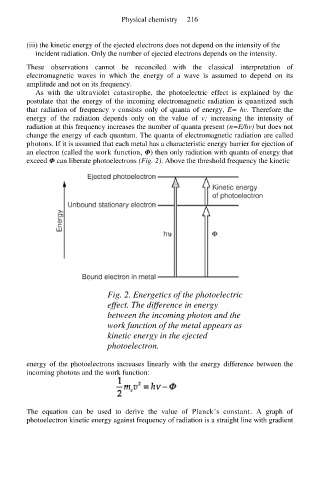
exceed Φ can liberate photoelectrons (Fig. 2). Above the threshold frequency the kinetic
Fig. 2. Energetics of the photoelectric
effect. The difference in energy
between the incoming photon and the
work function of the metal appears as
kinetic energy in the ejected
photoelectron.
energy of the photoelectrons increases linearly with the energy difference between the
incoming photons and the work function:
The equation can be used to derive the value of Planck’s constant. A graph of
photoelectron kinetic energy against frequency of radiation is a straight line with gradient