Page 542 - Instrumentation Reference Book 3E
P. 542
Detectors 525
output of the photomultiplier. Its higher density
than Nal (TI) allows the use of smaller crystals
but its low light output (8 percent of Nal (Tl)) is
a disadvantage. Against this it is non-liygro-
scopic, so it can he used with only light shielding.
CsF is a scintillator with a very fast decay time
(about 511s) but a low light output (18 percent of
in
t 1 r I ! I I I 1 I I I\ Nal ('I'l)). It also has been LIS~~ to~o~apl~ic
-BO-40 -20 0 20 40 60 EX2 100 120 140 160 scanners. nsefd
LiI (Eu) is n scintillator ~ar~icu~ar~y for
Temperaturs at cryzlal source ("C)
de~~tingneutrons~ as the lith~um content can
and
be changed by using enriched 6Li to enhance the
Csl
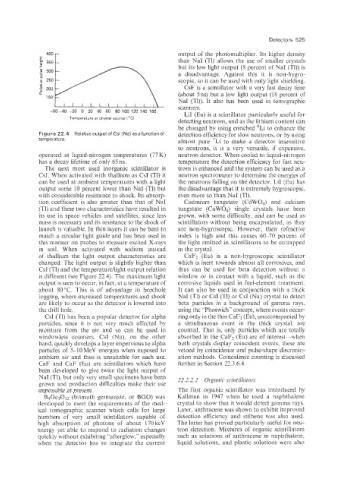
Figure 22.4 Relativ~ff~tpu~of (Na) asafun~tio~fff detection efficiency for slow neutrQns, or by using
~e~pera~~~~. almost pure 71ti to make a detecto- i~~se~isi~~~e
I
to neutrons, it is a very versatile, if expensive,
operated at li4u~d-ni€ro~en ~einperatures (77 fo neutron detector. When cooled to iiq~iid-nitroge~
the
has a decay lifetime of only 65 LIS. ~eiii~ra~ure detection efficiency for fast neu-
The next most used inorganic scintillator is trons is enhanced and the system can be used as a
Cs4. 'csiheii activated with thallium as GsI (33) it neutron spectrometer to deterniine the energies of
can be used at ambient temperatures with a light the neutrons falling on the detector. LiI (Ea) has
output some 10 percent lower than NaI (Tl) but the disadvantage that it is extremely hygroscopic,
with considerable resistance to shock. Its absorp- even more so than Nall (TI).
tion coefficieilt is also greater than that of NaT Cadmium tungstate (CdW04) and calcium
(Tlf and these two characteristics have resulted in tungstate (CaW04) single crystals have been
its use in space vehicles and satellites, since less grown, with some difficulty, and can be used as
mass is necessary and its resistance to the shock of scintillators without being encapsulated, as they
launch is valuable. In thin layers it can be bent to are non-hygroscopic. However, their refractive
match a circular light guide and has been used in index is high and this causes 60-70 percent of
this manner on probes to measure excited X-rays the light emitted in scintillatoss to be entrapped
in soil. When activated with sodium instead in the crystal.
of thallium the light output characteristics are CaFz (ELI) is a non-hygroscopic scintillator
charged. The light output is slightly higher than which is inert towards almost all corrosives, and
CsI (TI) and the temperatureilight output relation thus can be used for beta detection without a
is different (see Figure 22.4). The maximum light window or in contact with a liquid, such as the
output is seen to occur, in fact, at a temperature of corrosive liquids used in fuel-element treatment.
about 80°C. This is of advantage in borehole It can also be used in conjunction with a thick
iogging, where increased temperatures and shock NaI (Tl) or Csl (TI) or CsI (Na) crystal to detect
are likely to occur as the detector is lowered into beta particles in a background of ganima rays,
the drill hole. using the "Phoswich" concept, where events occur-
Csl (Tl) has been a popular detector for alpha ring only in the thin CaF2 (Eu), unaccompanied by
particlcs, since it is not very much affected by a simultaneous event in the thick crystal, are
moisture from the air and so can be used in counted. That is, only particles which are totally
windowless counters. CsI (Nd), on the other absorbed in the GaF2 (Eu) are of interest-when
hand. quickly develops a layer impervious to alpha both crystals display coincident events, these are
particles of 5-10 MeV energies when exposed to vetoed by coincidence and pulse-shape discrimin-
ambient air and thus is unsuitable for such use. ation methods. Coincidence counting is discussed
CaF and CaF (Eu) are scintillators which have further in Section 22.3.6.4.
been developed to give twice the light output of
NaI (Tl}, but only very small specimens have been 22.2.2.2 Orgmic scintillnrors
grown and production difficulties make their use
impossible at present. The first organic scintillator was inrroduced by
B4Ge3OI2 (bismuth gernianate, or BGO) was Kallrrian in 1947 when he used a naphthaiene
developed to meet the requirements of the med- crystal to show that it would detect gamma rays.
ical tomographic scanner wliich calfs for large L,ater, anthracene was shown to exhibit improved
numbers of very small scintillators capable of detection efficiency and stilbene was also used.
high absorption of photons of about 170keV The latter has proved particularly ~tseful for neu-
energy yet able to respond to radiation changes tron detection. Mixtures of organic scinti~l~~ors
quickly without e~hibiting "afterglow," especially such as solutions of anthracene in naph~hale~e,
when she detector has to integrate the current liquid solutions, and plastic solutions were also