Page 165 - Materials Chemistry, Second Edition
P. 165
L1644_C04.fm Page 137 Tuesday, October 21, 2003 3:13 PM
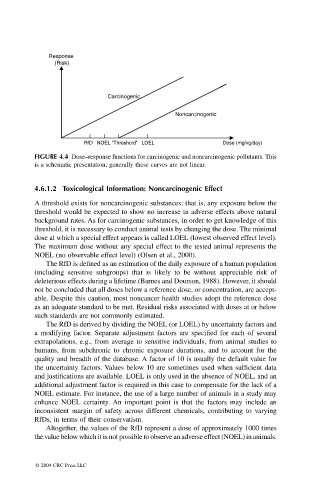
Response
(Risk)
Carcinogenic
Noncarcinogenic
RfD NOEL "Threshold" LOEL Dose (mg/kg/day)
FIGURE 4.4 Dose–response functions for carcinogenic and noncarcinogenic pollutants. This
is a schematic presentation; generally these curves are not linear.
4.6.1.2 Toxicological Information: Noncarcinogenic Effect
A threshold exists for noncarcinogenic substances; that is, any exposure below the
threshold would be expected to show no increase in adverse effects above natural
background rates. As for carcinogenic substances, in order to get knowledge of this
threshold, it is necessary to conduct animal tests by changing the dose. The minimal
dose at which a special effect appears is called LOEL (lowest observed effect level).
The maximum dose without any special effect to the tested animal represents the
NOEL (no observable effect level) (Olsen et al., 2000).
The RfD is defined as an estimation of the daily exposure of a human population
(including sensitive subgroups) that is likely to be without appreciable risk of
deleterious effects during a lifetime (Barnes and Dourson, 1988). However, it should
not be concluded that all doses below a reference dose, or concentration, are accept-
able. Despite this caution, most noncancer health studies adopt the reference dose
as an adequate standard to be met. Residual risks associated with doses at or below
such standards are not commonly estimated.
The RfD is derived by dividing the NOEL (or LOEL) by uncertainty factors and
a modifying factor. Separate adjustment factors are specified for each of several
extrapolations, e.g., from average to sensitive individuals, from animal studies to
humans, from subchronic to chronic exposure durations, and to account for the
quality and breadth of the database. A factor of 10 is usually the default value for
the uncertainty factors. Values below 10 are sometimes used when sufficient data
and justifications are available. LOEL is only used in the absence of NOEL, and an
additional adjustment factor is required in this case to compensate for the lack of a
NOEL estimate. For instance, the use of a large number of animals in a study may
enhance NOEL certainty. An important point is that the factors may include an
inconsistent margin of safety across different chemicals, contributing to varying
RfDs, in terms of their conservatism.
Altogether, the values of the RfD represent a dose of approximately 1000 times
the value below which it is not possible to observe an adverse effect (NOEL) in animals.
© 2004 CRC Press LLC