Page 156 - Introduction to Colloid and Surface Chemistry
P. 156
The solid-gas interface 145
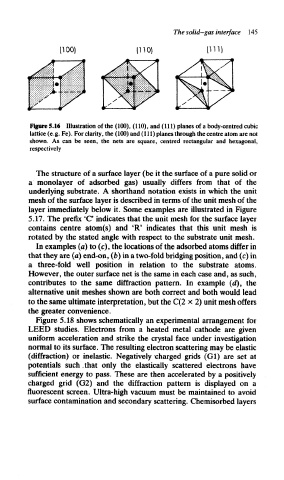
(111)
(100) (110)
Figure 5.16 Illustration of the (100), (110), and (111) planes of a body-centred cubic
lattice (e.g. Fe). For clarity, the (100) and (111) planes through the centre atom are not
shown. As can be seen, the nets are square, centred rectangular and hexagonal,
respectively
The structure of a surface layer (be it the surface of a pure solid or
a monolayer of adsorbed gas) usually differs from that of the
underlying substrate. A shorthand notation exists in which the unit
mesh of the surface layer is described in terms of the unit mesh of the
layer immediately below it. Some examples are illustrated in Figure
5.17. The prefix 'C' indicates that the unit mesh for the surface layer
contains centre atom(s) and 'R' indicates that this unit mesh is
rotated by the stated angle with respect to the substrate unit mesh.
In examples (a) to (c), the locations of the adsorbed atoms differ in
that they are (a) end-on, (b) in a two-fold bridging position, and (c) in
a three-fold well position in relation to the substrate atoms.
However, the outer surface net is the same in each case and, as such,
contributes to the same diffraction pattern. In example (</), the
alternative unit meshes shown are both correct and both would lead
to the same ultimate interpretation, but the C(2 x 2) unit mesh offers
the greater convenience.
Figure 5.18 shows schematically an experimental arrangement for
LEED studies. Electrons from a heated metal cathode are given
uniform acceleration and strike the crystal face under investigation
normal to its surface. The resulting electron scattering may be elastic
(diffraction) or inelastic. Negatively -charged grids (Gl) are set at
potentials such .that only the elastically scattered electrons have
sufficient energy to pass. These are then accelerated by a positively
charged grid (G2) and the diffraction pattern is displayed on a
fluorescent screen. Ultra-high vacuum must be maintained to avoid
surface contamination and secondary scattering. Chemisorbed layers