Page 35 - Introduction to Colloid and Surface Chemistry
P. 35
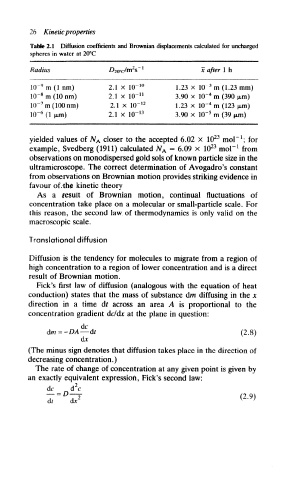
26 Kinetic properties
Table 2.1 Diffusion coefficients and Brownian displacements calculated for uncharged
spheres in water at 20°C
Radius D 2o°c/ m2s 1 He after I h
~
3
10~ 9 m (1 nm) 2.1 x 10~ 10 1.23 x 10~ m (1.23 mm)
4
8
10~ m (10 nm) 2.1 x 10~ n 3.90 x 10~ m (390 jim)
7
4
Hr rn(100nm) 2.1 x 10- 12 1.23 x 10~ m (123 pm)
5
10~ 6 (1 fxm) 2.1 x KT 13 3.90 x 10~ m (39 jutn)
23
yielded values of N A closer to the accepted 6.02 x 10 mol *; for
23 1
example, Svedberg (1911) calculated N A = 6.09 x 10 moP from
observations on monodispersed gold sols of known particle size in the
ultramicroscope. The correct determination of Avogadro's constant
from observations on Brownian motion provides striking evidence in
favour of .the kinetic theory
As a icsult of Brownian motion, continual fluctuations of
concentration take place on a molecular or small-particle scale. For
this reason, the second law of thermodynamics is only valid on the
macroscopic scale.
Translational diffusion
Diffusion is the tendency for molecules to migrate from a region of
high concentration to a region of lower concentration and is a direct
result of Brownian motion.
Pick's first law of diffusion (analogous with the equation of heat
conduction) states that the mass of substance dm diffusing in the x
direction in a time At across an area A is proportional to the
concentration gradient dc/dx at the plane in question:
dc
dm = -DA—dt (2.8) /
,
\
dx
(The minus sign denotes that diffusion takes place in the direction of
decreasing concentration.)
The rate of change of concentration at any given point is given by
an exactly equivalent expression, Pick's second law:
2
dc d c
= D r- (2 Q)
-
dt dx 2 lz v;