Page 38 - Introduction to Colloid and Surface Chemistry
P. 38
Kinetic properties 29
reciprocal of the ffictional ratio /// 0. Charge effects are discussed on
page 37.
Measurement of diffusion coefficients 27
Free boundary methods
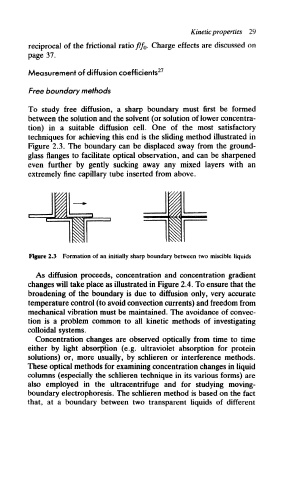
To study free diffusion, a sharp boundary must first be formed
between the solution and the solvent (or solution of lower concentra-
tion) in a suitable diffusion cell. One of the most satisfactory
techniques for achieving this end is the sliding method illustrated in
Figure 2.3. The boundary can be displaced away from the ground-
glass flanges to facilitate optical observation, and can be sharpened
even further by gently sucking away any mixed layers with an
extremely fine capillary tube inserted from above.
Figure 2.3 Formation of an initially sharp boundary between two miscible liquids
As diffusion proceeds, concentration and concentration gradient
changes will take place as illustrated in Figure 2.4. To ensure that the
broadening of the boundary is due to diffusion only, very accurate
temperature control (to avoid convection currents) and freedom from
mechanical vibration must be maintained. The avoidance of convec-
tion is a problem common to all kinetic methods of investigating
colloidal systems.
Concentration changes are observed optically from time to time
either by light absorption (e.g. ultraviolet absorption for protein
solutions) or, more usually, by schlieren or interference methods.
These optical methods for examining concentration changes in liquid
columns (especially the schlieren technique in its various forms) are
also employed in the ultracentrifuge and for studying moving-
boundary electrophoresis. The schlieren method is based on the fact
that, at a boundary between two transparent liquids of different