Page 205 - Introduction to Petroleum Engineering
P. 205
192 WELL COMPLETIONS
10.4 ACIDIZING
The next step after perforating is to clear debris and other damage for a cased and
cemented hole. If reservoir pressure is sufficiently high, opening the well and starting
production may be enough to clear the perforations. Acidizing is another option for
cleaning perforations. At any time in the life of a well, acids can be used to remove
mineral deposits in the well and the near‐well region in the formation that are lim-
iting production rate.
Four acids are often used. Hydrochloric (HCl), formic, and acetic acids attack
carbonate minerals such as calcite (CaCO ), dolomite (CaMg(CO ) ), and siderite
3
3 2
(FeCO ). Mixtures of hydrochloric and hydrofluoric (HF) acids are used in
3
treatments of silicate minerals such as quartz, various feldspars, and clays. The
volumetric amount of mineral that can be consumed by these acids depends on
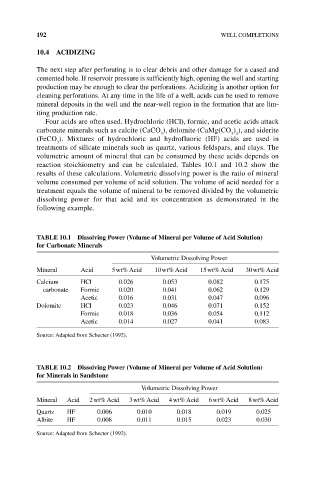
reaction stoichiometry and can be calculated. Tables 10.1 and 10.2 show the
results of these calculations. Volumetric dissolving power is the ratio of mineral
volume consumed per volume of acid solution. The volume of acid needed for a
treatment equals the volume of mineral to be removed divided by the volumetric
dissolving power for that acid and its concentration as demonstrated in the
following example.
TAbLE 10.1 Dissolving Power (Volume of Mineral per Volume of Acid Solution)
for Carbonate Minerals
Volumetric Dissolving Power
Mineral Acid 5 wt% Acid 10 wt% Acid 15 wt% Acid 30 wt% Acid
Calcium HCl 0.026 0.053 0.082 0.175
carbonate Formic 0.020 0.041 0.062 0.129
Acetic 0.016 0.031 0.047 0.096
Dolomite HCl 0.023 0.046 0.071 0.152
Formic 0.018 0.036 0.054 0.112
Acetic 0.014 0.027 0.041 0.083
Source: Adapted from Schecter (1992).
TAbLE 10.2 Dissolving Power (Volume of Mineral per Volume of Acid Solution)
for Minerals in Sandstone
Volumetric Dissolving Power
Mineral Acid 2 wt% Acid 3 wt% Acid 4 wt% Acid 6 wt% Acid 8 wt% Acid
Quartz HF 0.006 0.010 0.018 0.019 0.025
Albite HF 0.008 0.011 0.015 0.023 0.030
Source: Adapted from Schecter (1992).