Page 33 - Introduction to Petroleum Engineering
P. 33
PETROLEUM AND THE ENVIRONMENT 17
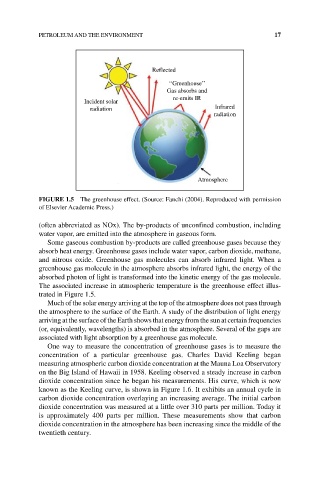
Reflected
‘‘Greenhouse’’
Gas absorbs and
re-emits IR
Incident solar
radiation Infrared
radiation
Atmosphere
FIGURE 1.5 The greenhouse effect. (Source: Fanchi (2004). Reproduced with permission
of Elsevier Academic Press.)
(often abbreviated as NOx). The by‐products of unconfined combustion, including
water vapor, are emitted into the atmosphere in gaseous form.
Some gaseous combustion by‐products are called greenhouse gases because they
absorb heat energy. Greenhouse gases include water vapor, carbon dioxide, methane,
and nitrous oxide. Greenhouse gas molecules can absorb infrared light. When a
greenhouse gas molecule in the atmosphere absorbs infrared light, the energy of the
absorbed photon of light is transformed into the kinetic energy of the gas molecule.
The associated increase in atmospheric temperature is the greenhouse effect illus-
trated in Figure 1.5.
Much of the solar energy arriving at the top of the atmosphere does not pass through
the atmosphere to the surface of the Earth. A study of the distribution of light energy
arriving at the surface of the Earth shows that energy from the sun at certain frequencies
(or, equivalently, wavelengths) is absorbed in the atmosphere. Several of the gaps are
associated with light absorption by a greenhouse gas molecule.
One way to measure the concentration of greenhouse gases is to measure the
concentration of a particular greenhouse gas. Charles David Keeling began
measuring atmospheric carbon dioxide concentration at the Mauna Loa Observatory
on the Big Island of Hawaii in 1958. Keeling observed a steady increase in carbon
dioxide concentration since he began his measurements. His curve, which is now
known as the Keeling curve, is shown in Figure 1.6. It exhibits an annual cycle in
carbon dioxide concentration overlaying an increasing average. The initial carbon
dioxide concentration was measured at a little over 310 parts per million. Today it
is approximately 400 parts per million. These measurements show that carbon
dioxide concentration in the atmosphere has been increasing since the middle of the
twentieth century.