Page 99 - Introduction to Petroleum Engineering
P. 99
84 MULTIPHASE FLOW
IFT has units of force per unit length, which is equivalent to energy per unit area.
IFT can refer to the force acting at the boundary of the interface between two phases or
to the energy needed to form the area within the boundary. IFT arises because of the
differences in molecular attractions that are experienced by molecules at the interface
between phases. Consider, for example, a water–oil interface. In water, molecules are
capable of hydrogen bonding with each other because of their polarity and shape. In
oil, molecules have little or no polarity, and bonding between molecules is weak. A
water molecule at the interface will feel strong attractive forces toward the other water
molecules in the water phase but not from the molecules in the oil phase. This difference
in attractions produces IFT. Some examples of IFT are listed in Table 5.1.
An important consequence of IFT is a difference in pressure between two adjacent
phases. That pressure difference is called capillary pressure p . It is proportional to
c
IFT and the inverse of curvature of the interface. For a spherical oil drop of radius r
surrounded by water, capillary pressure is the difference in pressure given by
2σ
p = p − p = ow (5.1)
cow o w r
where σ is the oil–water IFT. Pressure inside the drop of oil is higher than pressure
ow
in the surrounding water. Capillary pressure increases with decreasing size of pore
space. Shales have very small pores and capillary pressure can approach 1000 psi.
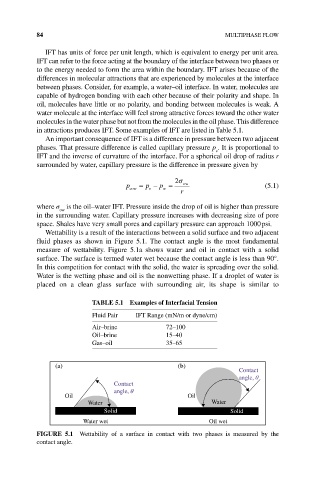
Wettability is a result of the interactions between a solid surface and two adjacent
fluid phases as shown in Figure 5.1. The contact angle is the most fundamental
measure of wettability. Figure 5.1a shows water and oil in contact with a solid
surface. The surface is termed water wet because the contact angle is less than 90°.
In this competition for contact with the solid, the water is spreading over the solid.
Water is the wetting phase and oil is the nonwetting phase. If a droplet of water is
placed on a clean glass surface with surrounding air, its shape is similar to
TABLE 5.1 Examples of Interfacial Tension
Fluid Pair IFT Range (mN/m or dyne/cm)
Air–brine 72–100
Oil–brine 15–40
Gas–oil 35–65
(a) (b)
Contact
angle,
Contact
angle,
Oil Oil
Water Water
Solid Solid
Water wet Oil wet
FIgURE 5.1 Wettability of a surface in contact with two phases is measured by the
contact angle.