Page 110 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 110
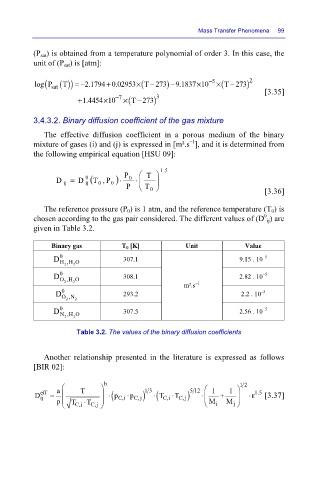
(P sat) is obtained from a temperature polynomial of order 3. In this case, the
unit of (P sat) is [atm]:
−
5
2
)
×
(T 273−
+
T
log P ()) =− 2.1794 0.02953× (T 273− ) 9.1837 10− × Mass Transfer Phenomena 99
( sat
[3.35]
×
+ 1.4454 10 − 7 × (T 273− ) 3
3.4.3.2. Binary diffusion coefficient of the gas mixture
The effective diffusion coefficient in a porous medium of the binary
–1
mixture of gases (i) and (j) is expressed in [m².s ], and it is determined from
the following empirical equation [HSU 09]:
D = D 0 (T P , )⋅ P 0 ⋅ T 5 . 1
0
ij
ij
0
P T 0 [3.36]
The reference pressure (P 0) is 1 atm, and the reference temperature (T 0) is
0
chosen according to the gas pair considered. The different values of (D ij) are
given in Table 3.2.
Binary gas T 0 [K] Unit Value
–5
D 0 H,H O 307.1 9.15 . 10
2
2
–5
D 0 O,H O 308.1 2.82 . 10
2
2
–1
m².s
–5
D 0 293.2 2.2 . 10
O,N
2 2
D 0 N,H O 307.5 2.56 . 10
–5
2
2
Table 3.2. The values of the binary diffusion coefficients
Another relationship presented in the literature is expressed as follows
[BIR 02]:
13
⋅
D eff = a T b ⋅ ( C,i p ⋅ C,j ) ( C,i T ⋅ C, ) j 5 12 ⋅ 1 + 1 12 ⋅ε 1.5 [3.37]
p
T
ij
p T T ⋅ M M j
C,i C,j i