Page 115 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 115
104 Introduction to Transfer Phenomena in PEM Fuel Cells
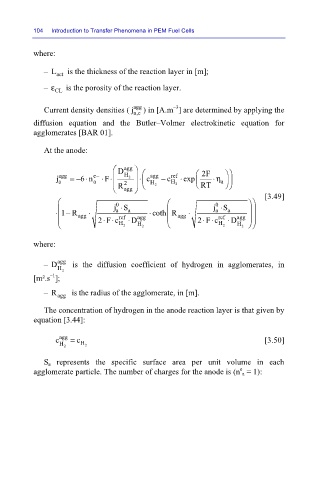
where:
is the thickness of the reaction layer in [m];
– L
act
– ε CL is the porosity of the reaction layer.
–3
Current density densities ( j agg ) in [A.m ] are determined by applying the
a,c
diffusion equation and the Butler–Volmer electrokinetic equation for
agglomerates [BAR 01].
At the anode:
D agg 2F
j agg =− 6 n ⋅ e− F ⋅ ⋅ H 2 ⋅ c agg − c ref 2 ⋅ exp RT ⋅η a
a
a
H
H
2
2
R agg
0 0 [3.49]
−
a
a
⋅ 1R ⋅ jS ⋅ a ⋅ coth R ⋅ jS ⋅ a
agg 2F c ref ⋅ D agg agg 2F c ⋅ ref ⋅ D agg
⋅⋅
⋅
H 2 H 2 H 2 H 2
where:
– D agg is the diffusion coefficient of hydrogen in agglomerates, in
H 2
–1
[m².s ];
– R agg is the radius of the agglomerate, in [m].
The concentration of hydrogen in the anode reaction layer is that given by
equation [3.44]:
c agg = c [3.50]
H 2 H 2
S a represents the specific surface area per unit volume in each
e
agglomerate particle. The number of charges for the anode is (n a = 1):