Page 117 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 117
106 Introduction to Transfer Phenomena in PEM Fuel Cells
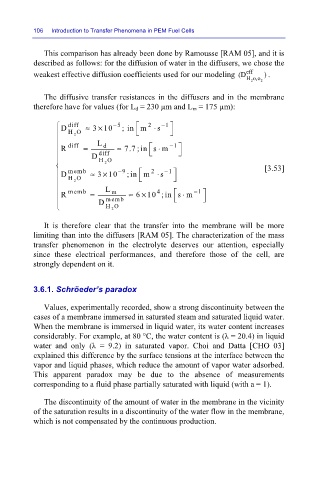
This comparison has already been done by Ramousse [RAM 05], and it is
described as follows: for the diffusion of water in the diffusers, we chose the
weakest effective diffusion coefficients used for our modeling (D eff ) .
Ho,o 2
2
The diffusive transfer resistances in the diffusers and in the membrane
therefore have for values (for L d = 230 µm and L m = 175 µm):
D diff ≈ 3 10 − 5 ; in m ⋅ s − 1
2
×
HO
2
L −
R diff = d ≈ 7.7 ; in s m 1
⋅
D diff
HO
2
[3.53]
2
D memb ≈ 3 10 − 9 ;in m ⋅ s − 1
×
HO
2
memb L m 4 − 1
⋅
×
R = memb ≈ 6 10 ; in s m
D HO
2
It is therefore clear that the transfer into the membrane will be more
limiting than into the diffusers [RAM 05]. The characterization of the mass
transfer phenomenon in the electrolyte deserves our attention, especially
since these electrical performances, and therefore those of the cell, are
strongly dependent on it.
3.6.1. Schröeder’s paradox
Values, experimentally recorded, show a strong discontinuity between the
cases of a membrane immersed in saturated steam and saturated liquid water.
When the membrane is immersed in liquid water, its water content increases
considerably. For example, at 80 °C, the water content is (λ = 20.4) in liquid
water and only (λ = 9.2) in saturated vapor. Choi and Datta [CHO 03]
explained this difference by the surface tensions at the interface between the
vapor and liquid phases, which reduce the amount of vapor water adsorbed.
This apparent paradox may be due to the absence of measurements
corresponding to a fluid phase partially saturated with liquid (with a = 1).
The discontinuity of the amount of water in the membrane in the vicinity
of the saturation results in a discontinuity of the water flow in the membrane,
which is not compensated by the continuous production.