Page 113 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 113
102 Introduction to Transfer Phenomena in PEM Fuel Cells
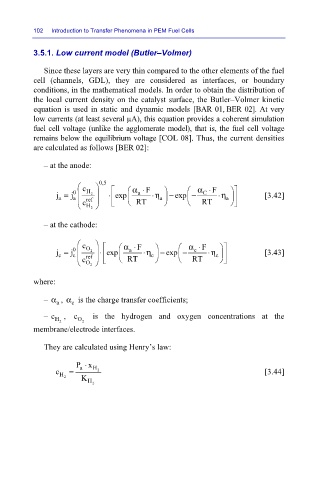
3.5.1. Low current model (Butler–Volmer)
Since these layers are very thin compared to the other elements of the fuel
cell (channels, GDL), they are considered as interfaces, or boundary
conditions, in the mathematical models. In order to obtain the distribution of
the local current density on the catalyst surface, the Butler–Volmer kinetic
equation is used in static and dynamic models [BAR 01, BER 02]. At very
low currents (at least several µA), this equation provides a coherent simulation
fuel cell voltage (unlike the agglomerate model), that is, the fuel cell voltage
remains below the equilibrium voltage [COL 08]. Thus, the current densities
are calculated as follows [BER 02]:
– at the anode:
c 0,5 α⋅ F α F ⋅
j = j 0 a H 2 ⋅ exp a ⋅η a − exp − C ⋅η a [3.42]
a
ref
c H 2 RT RT
– at the cathode:
c α⋅ F α F ⋅
j = j 0 c O 2 ⋅ exp a ⋅η c − exp − c ⋅η c [3.43]
c
ref
c O 2 RT RT
where:
– α , α is the charge transfer coefficients;
a
c
– c H 2 , c O 2 is the hydrogen and oxygen concentrations at the
membrane/electrode interfaces.
They are calculated using Henry’s law:
Px ⋅ H
a
c H 2 = K H 2 2 [3.44]