Page 66 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 66
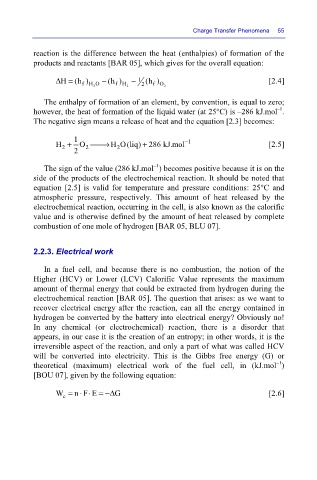
reaction is the difference between the heat (enthalpies) of formation of the
products and reactants [BAR 05], which gives for the overall equation:
Δ= (h) 2 − (h) 2 − 1 2 (h) 2 Charge Transfer Phenomena 55
H
[2.4]
fH O
fH
f O
The enthalpy of formation of an element, by convention, is equal to zero;
–1
however, the heat of formation of the liquid water (at 25°C) is –286 kJ.mol .
The negative sign means a release of heat and the equation [2.3] becomes:
H + 1 O ⎯⎯→ H O(liq) 286 kJ.mol − 1 [2.5]
+
2
2 2 2
–1
The sign of the value (286 kJ.mol ) becomes positive because it is on the
side of the products of the electrochemical reaction. It should be noted that
equation [2.5] is valid for temperature and pressure conditions: 25°C and
atmospheric pressure, respectively. This amount of heat released by the
electrochemical reaction, occurring in the cell, is also known as the calorific
value and is otherwise defined by the amount of heat released by complete
combustion of one mole of hydrogen [BAR 05, BLU 07].
2.2.3. Electrical work
In a fuel cell, and because there is no combustion, the notion of the
Higher (HCV) or Lower (LCV) Calorific Value represents the maximum
amount of thermal energy that could be extracted from hydrogen during the
electrochemical reaction [BAR 05]. The question that arises: as we want to
recover electrical energy after the reaction, can all the energy contained in
hydrogen be converted by the battery into electrical energy? Obviously no!
In any chemical (or electrochemical) reaction, there is a disorder that
appears, in our case it is the creation of an entropy; in other words, it is the
irreversible aspect of the reaction, and only a part of what was called HCV
will be converted into electricity. This is the Gibbs free energy (G) or
–1
theoretical (maximum) electrical work of the fuel cell, in (kJ.mol )
[BOU 07], given by the following equation:
W =⋅ ⋅ G [2.6]
n F E = −Δ
e