Page 70 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 70
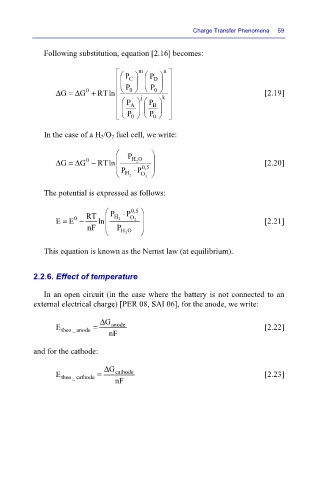
Following substitution, equation [2.16] becomes:
P m P n Charge Transfer Phenomena 59
C D
0
Δ= Δ G + RTln P 0 P 0 k [2.19]
G
P A j P B
P 0 P 0
In the case of a H 2/O 2 fuel cell, we write:
P
0
Δ= Δ G − RTln HO [2.20]
G
2
P P ⋅ 0,5
H 2 O 2
The potential is expressed as follows:
RT P P ⋅ 0,5
0
E = E − ln H 2 O 2 [2.21]
nF P
HO
2
This equation is known as the Nernst law (at equilibrium).
2.2.6. Effect of temperature
In an open circuit (in the case where the battery is not connected to an
external electrical charge) [PER 08, SAI 06], for the anode, we write:
Δ G
E theo _ anode = anode [2.22]
nF
and for the cathode:
Δ G
E = cathode [2.23]
theo _ cathode
nF