Page 67 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 67
56 Introduction to Transfer Phenomena in PEM Fuel Cells
and:
G Δ
−
Δ
where: Δ= H T S [2.7]
– n is the number of electrons exchanged in the elementary
electrochemical reaction, in the case of the hydrogen/oxygen reaction, n e = 2;
– F is the Faraday constant, electric charge of one mole of electrons, F =
–1
96 485 C.mol ;
– E is the electromotive force (emf) of the fuel cell.
In the same way, like formation enthalpies in equation [2.4], the
following equation represents the difference between entropy of products
and reactants:
Δ= fH O − (S ) 2 − 1 2 (S ) 2 [2.8]
S(S )
fH
f O
2
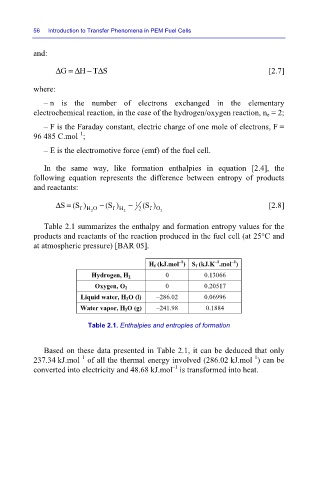
Table 2.1 summarizes the enthalpy and formation entropy values for the
products and reactants of the reaction produced in the fuel cell (at 25°C and
at atmospheric pressure) [BAR 05].
–1
–1
–1
H f (kJ.mol )S f (kJ.K .mol )
Hydrogen, H 2 0 0.13066
Oxygen, O 2 0 0.20517
Liquid water, H 2 O (l) –286.02 0.06996
Water vapor, H 2 O (g) –241.98 0.1884
Table 2.1. Enthalpies and entropies of formation
Based on these data presented in Table 2.1, it can be deduced that only
–1
–1
237.34 kJ.mol of all the thermal energy involved (286.02 kJ.mol ) can be
–1
converted into electricity and 48.68 kJ.mol is transformed into heat.