Page 84 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 84
Charge Transfer Phenomena 73
The value (J 0_C) is much smaller, by a factor of 10 , than the value of
(J O_a), it is therefore deemed that the overpotential at the anode is negligible;
hence, the equation [2.69] can be written as follows: 5
j
η act = Aln [2.70]
ac
b
with (A ac) expressed in (V) and characterized by the following equation:
A = A + A c [2.71]
a
ac
and (b) expressed in (mA/cm²) and characterized by the following relation:
A a A c
A
A
b = j 0_ a + j 0_ c [2.72]
a being the slope of the Tafel curve:
RT
A = [2.73]
α nF
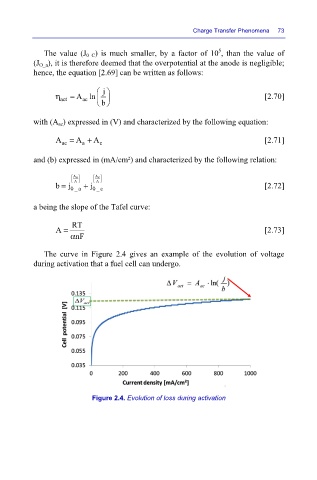
The curve in Figure 2.4 gives an example of the evolution of voltage
during activation that a fuel cell can undergo.
Figure 2.4. Evolution of loss during activation