Page 88 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 88
Charge Transfer Phenomena 77
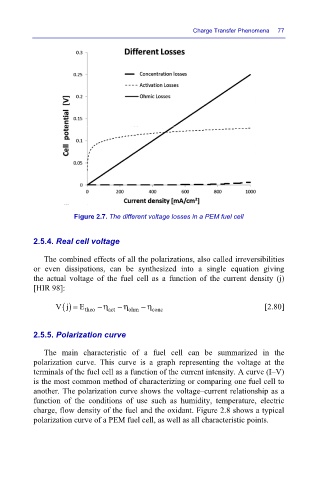
Figure 2.7. The different voltage losses in a PEM fuel cell
2.5.4. Real cell voltage
The combined effects of all the polarizations, also called irreversibilities
or even dissipations, can be synthesized into a single equation giving
the actual voltage of the fuel cell as a function of the current density (j)
[HIR 98]:
() E=
Vj theo − η act − η ohm − η conc [2.80]
2.5.5. Polarization curve
The main characteristic of a fuel cell can be summarized in the
polarization curve. This curve is a graph representing the voltage at the
terminals of the fuel cell as a function of the current intensity. A curve (I–V)
is the most common method of characterizing or comparing one fuel cell to
another. The polarization curve shows the voltage–current relationship as a
function of the conditions of use such as humidity, temperature, electric
charge, flow density of the fuel and the oxidant. Figure 2.8 shows a typical
polarization curve of a PEM fuel cell, as well as all characteristic points.