Page 99 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 99
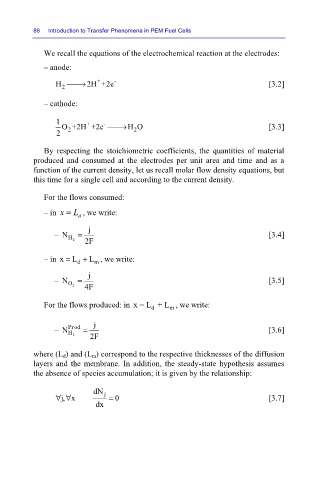
88 Introduction to Transfer Phenomena in PEM Fuel Cells
We recall the equations of the electrochemical reaction at the electrodes:
– anode:
+
H ⎯⎯→ 2H +2e - [3.2]
2
– cathode:
1 O+2H +2e ⎯⎯→ H O [3.3]
+
-
2 2 2
By respecting the stoichiometric coefficients, the quantities of material
produced and consumed at the electrodes per unit area and time and as a
function of the current density, let us recall molar flow density equations, but
this time for a single cell and according to the current density.
For the flows consumed:
– in x = L d , we write:
j
– N H 2 = 2F [3.4]
– in x = L + L , we write:
d
m
j
– N O 2 = 4F [3.5]
For the flows produced: in x = L + L , we write:
m
d
j
– N Prod = 2F [3.6]
H
2
where (L d) and (L m) correspond to the respective thicknesses of the diffusion
layers and the membrane. In addition, the steady-state hypothesis assumes
the absence of species accumulation; it is given by the relationship:
dN
∀∀ j = 0 [3.7]
j, x
dx