Page 98 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 98
Mass Transfer Phenomena 87
The convective forces that dominate the mass transfer in the flow
channels are mainly imposed by the flow of fuel (hydrogen), while the flow
conditions of the oxidant can be controlled by the user. High reagent flow
rates can provide good distribution at the catalytic sites of the electrodes, but
this can also cause problems in the overall fuel cell and can even damage the
polymer membrane. The diffusive forces acting on the surface of the
electrodes are supported by convective forces upstream (from the flow
channels). Indeed, the velocity of the reagents decreases along its path to the
diffusion layers where diffusion begins. The consumption of gaseous
reactants and the production of water at the cathode are a function of the
flow of electrons passing through the external circuit, and therefore of the
intensity, through the electrochemical reactions. In other words, the material
flow densities are directly related to the current density delivered by the fuel
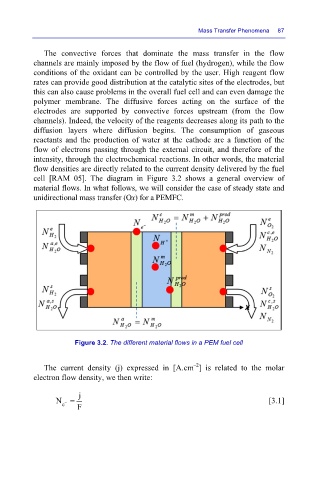
cell [RAM 05]. The diagram in Figure 3.2 shows a general overview of
material flows. In what follows, we will consider the case of steady state and
unidirectional mass transfer (Ox) for a PEMFC.
Figure 3.2. The different material flows in a PEM fuel cell
–2
The current density (j) expressed in [A.cm ] is related to the molar
electron flow density, we then write:
j
N − = [3.1]
e F