Page 138 - Introduction to chemical reaction engineering and kinetics
P. 138
120 Chapter 6: Fundamentals of Reaction Rates
6.2.2 General Requirements for Elementary Chemical Reactions
The requirements for a reaction to occur are:
(1) The reaction partners must encounter one another.
(2) The encounter must be successful. This in turn requires:
(i) the geometry of the encounter to be correct (e.g., the atoms in the proper
position to form the new bonds) and,
(ii) sufficient energy to be available to overcome any energy barriers to this
transformation.
The simple theories of reaction rates involve applying basic physical chemistry knowl-
edge to calculate or estimate the rates of successful molecular encounters. In Section
6.3 we present important results from physical chemistry for this purpose; in subse-
quent sections, we show how they are used to build rate theories, construct rate laws,
and estimate the values of rate constants for elementary reactions.
6.3 ENERGY IN MOLECULES
Energy in molecules, as in macroscopic objects, can be divided into potential energy
(the energy which results from their position at rest) and kinetic energy (energy asso-
ciated with motion). Potential energy in our context deals with the energy associated
with chemical bonding. The changes in bond energy often produce energy barriers to
reaction as the atoms rearrange. The kinetic energy of a group of molecules governs
(1) how rapidly reactants encounter one another, and (2) how much energy is available
in the encounter to surmount any barriers to reaction. Research has led to a detailed
understanding of how these factors influence the rates of elementary reactions, and
was recognized by the award of the Nobel prize in chemistry to Lee, Herschbach, and
Polanyi in 1986.
6.3.1 Potential Energy in Molecules-Requirements for Reaction
6.3.1.1 Diatomic Molecules
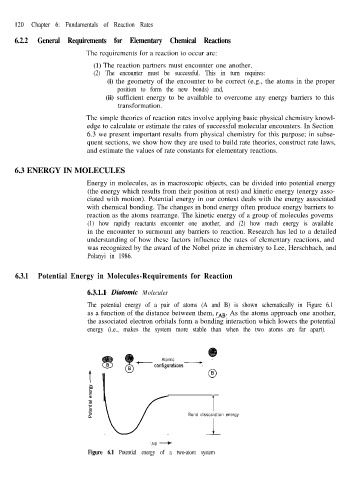
The potential energy of a pair of atoms (A and B) is shown schematically in Figure 6.1
as a function of the distance between them, rAB. As the atoms approach one another,
the associated electron orbitals form a bonding interaction which lowers the potential
energy (i.e., makes the system more stable than when the two atoms are far apart).
Atomic
a configurations-
@
t
Bond dissociation energy
-l
‘AB -
Figure 6.1 Potential energy of a two-atom system